当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
3-Substituted 2-Aminonaphthalene Photocages for Carboxylic Acids and Alcohols; Decaging Mechanism and Potential Applications in Synthesis
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-10-13 , DOI: 10.1021/acs.joc.3c01678 Vilma Lovrinčević 1 , Yan Guo 2 , Dragana Vuk 1 , Irena Škorić 1 , Jiani Ma 2 , Nikola Basarić 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-10-13 , DOI: 10.1021/acs.joc.3c01678 Vilma Lovrinčević 1 , Yan Guo 2 , Dragana Vuk 1 , Irena Škorić 1 , Jiani Ma 2 , Nikola Basarić 3
Affiliation
|
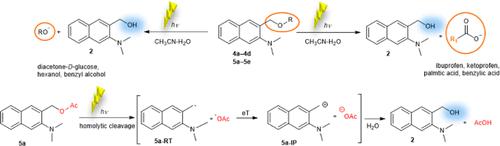
3-Hydroxymethyl-2-aminonaphthalene photocage (photoremovable protecting group) 2 was synthesized and transformed to different ethers and esters to investigate the applicability to decage alcohols and carboxylic acids, respectively. The photoelimination of carboxylic acids takes place relatively efficiently (ΦR = 0.11) upon excitation with near-visible light, contrary to the elimination of alcohols. The scope of the decaging of both alcohols and esters was demonstrated on several examples, including aliphatic and aromatic substrates, carbohydrates, and nonsteroidal anti-inflammatory drugs. The photophysical properties of the photocage and its models, methyl ether 4a and acetyl ester 5a, were investigated. The fluorescence quantum yields (Φf = 0.40–0.002) were found to be reversely proportional to the efficiency of elimination of OH, alcohols, or carboxylic acids. The decaging photochemical reaction mechanism was investigated experimentally by transient absorption techniques with time scales from femtoseconds to seconds and computationally on the TD-DFT level of theory. The photoelimination of carboxylates takes place directly in the singlet excited state by a homolytic cleavage producing a radical pair within 1 ns. The subsequent electron transfer gives rise to aminonaphthalene carbocation and the carboxylate. A wide scope of substrates that can be decaged relatively efficiently with near-visible light and the chromo-orthogonal compatibility of aminonaphthalene and aniline derivatives render these photocages potentially applicable in organic synthesis or biology.
中文翻译:

用于羧酸和醇的 3-取代 2-氨基萘光笼;分解机制及其在合成中的潜在应用
合成了3-羟甲基-2-氨基萘光笼(光可去除保护基)2,并将其转化为不同的醚和酯,以分别研究其对醇和羧酸的脱笼适用性。在近可见光激发下,羧酸的光消除相对有效(Φ R = 0.11),这与醇的消除相反。几个例子证明了醇和酯的分解范围,包括脂肪族和芳香族底物、碳水化合物和非甾体抗炎药。研究了光笼及其模型甲醚4a和乙酰酯5a的光物理性质。发现荧光量子产率 (Φ f = 0.40–0.002) 与 OH、醇或羧酸的消除效率成反比。通过瞬态吸收技术,时间尺度从飞秒到秒,并在 TD-DFT 理论水平上进行计算,对脱腐光化学反应机理进行了实验研究。羧酸盐的光消除直接发生在单线激发态,通过均裂在 1 ns 内产生自由基对。随后的电子转移产生氨基萘碳正离子和羧酸盐。可以用近可见光相对有效地脱笼的多种底物以及氨基萘和苯胺衍生物的色正交相容性使得这些光笼潜在地适用于有机合成或生物学。
更新日期:2023-10-13
中文翻译:

用于羧酸和醇的 3-取代 2-氨基萘光笼;分解机制及其在合成中的潜在应用
合成了3-羟甲基-2-氨基萘光笼(光可去除保护基)2,并将其转化为不同的醚和酯,以分别研究其对醇和羧酸的脱笼适用性。在近可见光激发下,羧酸的光消除相对有效(Φ R = 0.11),这与醇的消除相反。几个例子证明了醇和酯的分解范围,包括脂肪族和芳香族底物、碳水化合物和非甾体抗炎药。研究了光笼及其模型甲醚4a和乙酰酯5a的光物理性质。发现荧光量子产率 (Φ f = 0.40–0.002) 与 OH、醇或羧酸的消除效率成反比。通过瞬态吸收技术,时间尺度从飞秒到秒,并在 TD-DFT 理论水平上进行计算,对脱腐光化学反应机理进行了实验研究。羧酸盐的光消除直接发生在单线激发态,通过均裂在 1 ns 内产生自由基对。随后的电子转移产生氨基萘碳正离子和羧酸盐。可以用近可见光相对有效地脱笼的多种底物以及氨基萘和苯胺衍生物的色正交相容性使得这些光笼潜在地适用于有机合成或生物学。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号