Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2023-10-11 , DOI: 10.1016/j.jechem.2023.09.034
Daniela M. Josepetti , Bianca P. Sousa , Simone A. J. Rodrigues , Renato G. Freitas , Gustavo Doubek
|
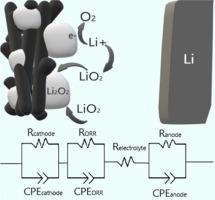
Lithium-oxygen batteries are a promising technology because they can greatly surpass the energy density of lithium-ion batteries. However, this theoretical characteristic has not yet been converted into a real device with high cyclability. Problems with air contamination, metallic lithium reactivity, and complex discharge and charge reactions are the main issues for this technology. A fast and reversible oxygen reduction reaction (ORR) is crucial for good performance of secondary batteries’, but the partial knowledge of its mechanisms, especially when devices are concerned, hinders further development. From this perspective, the present work uses operando Raman experiments and electrochemical impedance spectroscopy (EIS) to assess the first stages of the discharge processes in porous carbon electrodes, following their changes cycle by cycle at initial operation. A growth kinetic formation of the discharge product signal (Li2O2) was observed with operando Raman, indicating a first-order reaction and enabling an analysis by a microkinetic model. The solution mechanism in the evaluated system was ascribed for an equivalent circuit with three time constants. While the time constant for the anode interface reveals to remain relatively constant after the first discharge, its surface seemed to be more non-uniform. The model indicated that the reaction occurs at the Li2O2 surface, decreasing the associated resistance during the initial discharge phase. Furthermore, the growth of Li2O2 forms a hetero-phase between Li2O2/electrolyte, while creating a more compact and homogeneous on the Li2O2/cathode surface. The methodology here described thus offers a way of directly probing changes in surface chemistry evolution during cycling from a device through EIS analysis.
中文翻译:

Li-O2电池中氧还原反应过程中Li2O2形成的初始阶段:Li2O2在器件内电荷转移反应中的重要性
锂氧电池是一项很有前途的技术,因为它们可以大大超越锂离子电池的能量密度。然而,这一理论特性尚未转化为具有高循环性能的实际器件。空气污染、金属锂反应性以及复杂的放电和充电反应等问题是该技术的主要问题。快速且可逆的氧还原反应(ORR)对于二次电池的良好性能至关重要,但对其机制的部分了解,特别是在涉及设备时,阻碍了进一步的发展。从这个角度来看,目前的工作使用操作拉曼实验和电化学阻抗谱(EIS)来评估多孔碳电极放电过程的第一阶段,跟踪它们在初始操作中逐周期的变化。使用操作拉曼观察到放电产物信号(Li 2 O 2 )的生长动力学形成,表明一级反应并能够通过微动力学模型进行分析。评估系统中的解决机制归因于具有三个时间常数的等效电路。虽然阳极界面的时间常数在第一次放电后保持相对恒定,但其表面似乎更加不均匀。该模型表明反应发生在Li 2 O 2表面,从而降低了初始放电阶段的相关电阻。此外,Li 2 O 2的生长在Li 2 O 2 /电解质之间形成异相,同时在Li 2 O 2 /阴极表面上产生更致密和均匀的结构。因此,这里描述的方法提供了一种通过 EIS 分析直接探测设备循环过程中表面化学演化变化的方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号