当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulated Interfacial Proton and Water Activity Enhances Mn2+/MnO2 Platform Voltage and Energy Efficiency
ACS Energy Letters ( IF 19.3 ) Pub Date : 2023-10-13 , DOI: 10.1021/acsenergylett.3c01354
Xinzhe Xue 1 , Zhen Liu 1 , Samuel Eisenberg 1 , Qiu Ren 1 , Dun Lin 1 , Emma Coester 1 , Heng Zhang 1 , Jin Zhong Zhang 1 , Xiao Wang 1 , Yat Li 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2023-10-13 , DOI: 10.1021/acsenergylett.3c01354
Xinzhe Xue 1 , Zhen Liu 1 , Samuel Eisenberg 1 , Qiu Ren 1 , Dun Lin 1 , Emma Coester 1 , Heng Zhang 1 , Jin Zhong Zhang 1 , Xiao Wang 1 , Yat Li 1
Affiliation
|
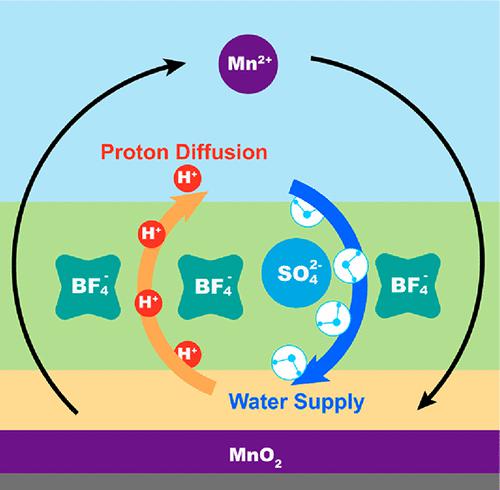
Electrolytic MnO2 batteries store charges via the Mn2+/MnO2 two-electron transfer process with higher capacity and voltage than conventional one-electron (Zn2+ or H+) intercalation reactions. Yet, the opposite effect of interfacial H+ on the dissolution/deposition processes and the role of interfacial H2O are rarely discussed. Here we introduce tetrafluoroborate (BF4–) into the sulfate-based electrolyte to regulate interfacial H+ and H2O activity. First, BF4– hydrolysis increases the electrolyte’s acidity, promoting MnO2 dissolution. Second, BF4– forms H-bond networks with interfacial H2O that assist H+ diffusion while retaining a sufficient H2O supply to facilitate MnO2 deposition. As a result, the cathode-free Zn//MnO2 electrolytic cell achieves a high platform of ∼1.92 V and energy efficiency of ∼84.23%. Significantly, the cell delivers 1000 cycles at 1 C with ∼100% Coulombic efficiency and a high energy efficiency retention of 93.65%. Our findings disclose a new strategy to promote Mn2+/MnO2 platform voltage and energy efficiency.
中文翻译:

调节界面质子和水活度提高 Mn2+/MnO2 平台电压和能源效率
电解MnO 2电池通过Mn 2+ /MnO 2双电子转移过程存储电荷,比传统的单电子(Zn 2+或H +)插层反应具有更高的容量和电压。然而,界面H +对溶解/沉积过程的相反影响以及界面H 2 O的作用却很少被讨论。在这里,我们将四氟硼酸盐(BF 4 –)引入硫酸盐基电解质中以调节界面H +和H 2 O活性。首先,BF 4 -水解增加了电解质的酸度,促进了MnO 2的溶解。其次,BF 4 –与界面H 2 O形成氢键网络,有助于H +扩散,同时保留足够的H 2 O供应以促进MnO 2沉积。结果,无阴极Zn//MnO 2电解池实现了~1.92 V的高平台和~84.23%的能源效率。值得注意的是,该电池在 1 C 下可循环 1000 次,库仑效率约为 100%,能效保持率高达 93.65%。我们的研究结果揭示了一种提高 Mn 2+ /MnO 2平台电压和能源效率的新策略。
更新日期:2023-10-13
中文翻译:

调节界面质子和水活度提高 Mn2+/MnO2 平台电压和能源效率
电解MnO 2电池通过Mn 2+ /MnO 2双电子转移过程存储电荷,比传统的单电子(Zn 2+或H +)插层反应具有更高的容量和电压。然而,界面H +对溶解/沉积过程的相反影响以及界面H 2 O的作用却很少被讨论。在这里,我们将四氟硼酸盐(BF 4 –)引入硫酸盐基电解质中以调节界面H +和H 2 O活性。首先,BF 4 -水解增加了电解质的酸度,促进了MnO 2的溶解。其次,BF 4 –与界面H 2 O形成氢键网络,有助于H +扩散,同时保留足够的H 2 O供应以促进MnO 2沉积。结果,无阴极Zn//MnO 2电解池实现了~1.92 V的高平台和~84.23%的能源效率。值得注意的是,该电池在 1 C 下可循环 1000 次,库仑效率约为 100%,能效保持率高达 93.65%。我们的研究结果揭示了一种提高 Mn 2+ /MnO 2平台电压和能源效率的新策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号