Basic Research in Cardiology ( IF 7.5 ) Pub Date : 2023-10-09 , DOI: 10.1007/s00395-023-01014-0 Dina Xie 1 , Hanliang Guo 1 , Mingbiao Li 1 , Liqun Jia 1 , Hao Zhang 1 , Degang Liang 1 , Naishi Wu 1 , Zequan Yang 2 , Yikui Tian 1, 3
|
The spleen contributes importantly to myocardial ischemia/reperfusion (MI/R) injury. Nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) recruits inflammasomes, initiating inflammatory responses and mediating tissue injury. We hypothesize that myocardial cell-free DNA (cfDNA) activates the splenic NLRP3 inflammasome during early reperfusion, increases systemic inflammatory response, and exacerbates myocardial infarct. Mice were subjected to 40 min of ischemia followed by 0, 1, 5, or 15 min, or 24 h of reperfusion. Splenic leukocyte adoptive transfer was performed by injecting isolated splenocytes to mice with splenectomy performed prior to left coronary artery occlusion. CY-09 (4 mg/kg) was administered 5 min before reperfusion. During post-ischemic reperfusion, splenic protein levels of NLRP3, cleaved caspase-1, and interleukin-1β (IL-1β) were significantly elevated and peaked (2.1 ± 0.2-, 3.4 ± 0.4-, and 3.2 ± 0.2-fold increase respectively, p < 0.05) within 5 min of reperfusion. In myocardial tissue, NLRP3 was not upregulated until 24 h after reperfusion. Suppression by CY09, a specific NLRP3 inflammasome inhibitor, or deficiency of NLRP3 significantly reduced myocardial infarct size (17.3% ± 4.2% and 33.2% ± 1.8% decrease respectively, p < 0.01). Adoptive transfer of NLRP3−/− splenocytes to WT mice significantly decreased infarct size compared to transfer of WT splenocytes (19.1% ± 2.8% decrease, p < 0.0001). NLRP3 was mainly activated at 5 min after reperfusion in CD11b+ and LY6G− splenocytes, which significantly increased during reperfusion (24.8% ± 0.7% vs.14.3% ± 0.6%, p < 0.0001). The circulating cfDNA level significantly increased in patients undergoing cardiopulmonary bypass (CPB) (43.3 ± 5.3 ng/mL, compared to pre-CPB 23.8 ± 3.5 ng/mL, p < 0.01). Mitochondrial cfDNA (mt-cfDNA) contributed to NLRP3 activation in macrophages (2.1 ± 0.2-fold increase, p < 0.01), which was inhibited by a Toll-like receptor 9(TLR9) inhibitor. The NLRP3 inflammasome in splenic monocytes is activated and mediates the inflammatory response shortly after reperfusion onset, exacerbating MI/R injury in mt-cfDNA/TLR9-dependent fashion.
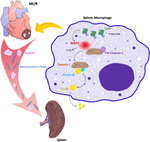
Graphical abstract
The schema reveals splenic NLRP3 mediates the inflammatory response in macrophages and exacerbates MI/R in a mitochondrial cfDNA/ TLR9-dependent fashion.
中文翻译:

脾单核细胞以线粒体无细胞DNA-TLR9-NLRP3依赖性方式介导炎症反应并加剧心肌缺血/再灌注损伤
脾脏对心肌缺血/再灌注(MI/R)损伤有重要影响。核苷酸结合寡聚结构域样受体家族pyrin结构域3 (NLRP3) 招募炎症小体,引发炎症反应并介导组织损伤。我们假设心肌游离DNA(cfDNA)在再灌注早期激活脾NLRP3炎性小体,增加全身炎症反应,并加剧心肌梗死。小鼠经历 40 分钟的缺血,然后再灌注 0、1、5、15 分钟或 24 小时。通过将分离的脾细胞注射到在左冠状动脉闭塞之前进行脾切除的小鼠体内来进行脾白细胞过继转移。再灌注前 5 分钟给予 CY-09 (4 mg/kg)。缺血后再灌注期间,脾脏中 NLRP3、裂解型 caspase-1 和白细胞介素 1β (IL-1β) 的蛋白水平显着升高并达到峰值(分别增加 2.1 ± 0.2、3.4 ± 0.4 和 3.2 ± 0.2 倍) , p < 0.05)再灌注 5 分钟内。在心肌组织中,NLRP3直到再灌注后24小时才上调。 CY09(一种特定的 NLRP3 炎性体抑制剂)的抑制或 NLRP3 的缺乏可显着减少心肌梗死面积(分别减少 17.3% ± 4.2% 和 33.2% ± 1.8%, p < 0.01)。与 WT 脾细胞移植相比,NLRP3 -/−脾细胞过继转移至 WT 小鼠可显着减少梗死面积(减少 19.1% ± 2.8%, p < 0.0001)。 NLRP3主要在再灌注后5分钟在CD11b +和LY6G -脾细胞中激活,在再灌注期间显着增加(24.8% ± 0.7% vs.14.3% ± 0.6%, p < 0.0001)。 接受体外循环 (CPB) 的患者的循环 cfDNA 水平显着升高(43.3 ± 5.3 ng/mL,相比之下,CPB 前为 23.8 ± 3.5 ng/mL, p < 0.01)。线粒体 cfDNA (mt-cfDNA) 有助于巨噬细胞中 NLRP3 的激活(增加 2.1 ± 0.2 倍, p < 0.01),而这种激活可被 Toll 样受体 9 (TLR9) 抑制剂抑制。再灌注开始后不久,脾单核细胞中的 NLRP3 炎症小体被激活并介导炎症反应,以 mt-cfDNA/TLR9 依赖性方式加剧 MI/R 损伤。
图形概要
该图显示脾 NLRP3 介导巨噬细胞中的炎症反应,并以线粒体 cfDNA/TLR9 依赖性方式加剧 MI/R。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号