Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ag-Co3O4-CoOOH-Nanowires Tandem Catalyst for Efficient Electrocatalytic Conversion of Nitrate to Ammonia at Low Overpotential via Triple Reactions
Advanced Science ( IF 14.3 ) Pub Date : 2023-10-11 , DOI: 10.1002/advs.202303789 Shilu Wu 1 , Yingyang Jiang 1 , Wenjie Luo 1 , Peng Xu 1 , Longlong Huang 1 , Yiwen Du 1 , Hui Wang 1 , Xuemei Zhou 1 , Yongjie Ge 1 , Jinjie Qian 1 , Huagui Nie 1 , Zhi Yang 1
Advanced Science ( IF 14.3 ) Pub Date : 2023-10-11 , DOI: 10.1002/advs.202303789 Shilu Wu 1 , Yingyang Jiang 1 , Wenjie Luo 1 , Peng Xu 1 , Longlong Huang 1 , Yiwen Du 1 , Hui Wang 1 , Xuemei Zhou 1 , Yongjie Ge 1 , Jinjie Qian 1 , Huagui Nie 1 , Zhi Yang 1
Affiliation
|
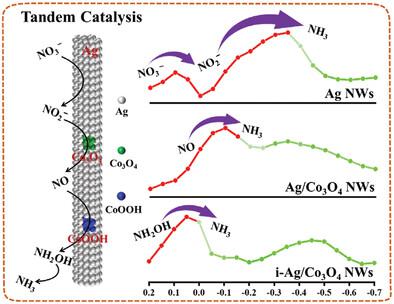
The electrocatalytic conversion of nitrate (NO3‾) to NH3 (NO3RR) offers a promising alternative to the Haber–Bosch process. However, the overall kinetic rate of NO3RR is plagued by the complex proton-assisted multiple-electron transfer process. Herein, Ag/Co3O4/CoOOH nanowires (i-Ag/Co3O4 NWs) tandem catalyst is designed to optimize the kinetic rate of intermediate reaction for NO3RR simultaneously. The authors proved that NO3‾ ions are reduced to NO2‾ preferentially on Ag phases and then NO2‾ to NO on Co3O4 phases. The CoOOH phases catalyze NO reduction to NH3 via NH2OH intermediate. This unique catalyst efficiently converts NO3‾ to NH3 through a triple reaction with a high Faradaic efficiency (FE) of 94.3% and a high NH3 yield rate of 253.7 μmol h−1 cm−2 in 1 M KOH and 0.1 M KNO3 solution at ‒0.25 V versus RHE. The kinetic studies demonstrate that converting NH2OH into NH3 is the rate-determining step (RDS) with an energy barrier of 0.151 eV over i-Ag/Co3O4 NWs. Further applying i-Ag/Co3O4 NWs as the cathode material, a novel Zn-nitrate battery exhibits a power density of 2.56 mW cm−2 and an FE of 91.4% for NH3 production.
中文翻译:

Ag-Co3O4-CoOOH-纳米线串联催化剂通过三重反应在低过电势下将硝酸盐高效电催化转化为氨
硝酸盐 (NO 3 ‾) 到 NH 3 (NO 3 RR) 的电催化转化为 Haber-Bosch 工艺提供了一种有前景的替代方案。然而,NO 3 RR的整体动力学速率受到复杂的质子辅助多电子转移过程的困扰。本文设计了Ag/Co 3 O 4 /CoOOH纳米线(i-Ag/Co 3 O 4 NWs)串联催化剂来同时优化NO 3 RR 中间反应的动力学速率。作者证明,NO 3 + 离子在Ag 相上优先还原为NO 2 + ,然后在Co 3 O 4相上将NO 2 + 还原为NO 。CoOOH 相通过NH 2 OH中间体催化NO还原为NH 3。这种独特的催化剂通过三重反应有效地将NO 3 ‾转化为NH 3 , 在1 M KOH和0.1 M KNO中法拉第 效率(FE)高达94.3%,NH 3 产率高达253.7 μmol h -1 cm -2 3 解决方案,相对于 RHE 为 −0.25 V。动力学研究表明,将 NH 2 OH 转化为 NH 3是 i-Ag/Co 3 O 4 NW 上的速率决定步骤 (RDS),其能垒为 0.151 eV 。进一步应用i-Ag/Co 3 O 4 NWs作为正极材料,新型硝酸锌电池的功率密度为2.56 mW cm -2 ,NH 3 生产 的FE为91.4% 。
更新日期:2023-10-11
中文翻译:

Ag-Co3O4-CoOOH-纳米线串联催化剂通过三重反应在低过电势下将硝酸盐高效电催化转化为氨
硝酸盐 (NO 3 ‾) 到 NH 3 (NO 3 RR) 的电催化转化为 Haber-Bosch 工艺提供了一种有前景的替代方案。然而,NO 3 RR的整体动力学速率受到复杂的质子辅助多电子转移过程的困扰。本文设计了Ag/Co 3 O 4 /CoOOH纳米线(i-Ag/Co 3 O 4 NWs)串联催化剂来同时优化NO 3 RR 中间反应的动力学速率。作者证明,NO 3 + 离子在Ag 相上优先还原为NO 2 + ,然后在Co 3 O 4相上将NO 2 + 还原为NO 。CoOOH 相通过NH 2 OH中间体催化NO还原为NH 3。这种独特的催化剂通过三重反应有效地将NO 3 ‾转化为NH 3 , 在1 M KOH和0.1 M KNO中法拉第 效率(FE)高达94.3%,NH 3 产率高达253.7 μmol h -1 cm -2 3 解决方案,相对于 RHE 为 −0.25 V。动力学研究表明,将 NH 2 OH 转化为 NH 3是 i-Ag/Co 3 O 4 NW 上的速率决定步骤 (RDS),其能垒为 0.151 eV 。进一步应用i-Ag/Co 3 O 4 NWs作为正极材料,新型硝酸锌电池的功率密度为2.56 mW cm -2 ,NH 3 生产 的FE为91.4% 。































 京公网安备 11010802027423号
京公网安备 11010802027423号