当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biochemical and Cellular Characterization of the Function of Fluorophosphonate-Binding Hydrolase H (FphH) in Staphylococcus aureus Support a Role in Bacterial Stress Response
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2023-10-12 , DOI: 10.1021/acsinfecdis.3c00246
Matthias Fellner 1 , Annabel Walsh 1 , Stephen Dela Ahator 2 , Nadia Aftab 2 , Ben Sutherland 3 , Eng W Tan 3 , Alexander T Bakker 4 , Nathaniel I Martin 5 , Mario van der Stelt 4 , Christian S Lentz 2
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2023-10-12 , DOI: 10.1021/acsinfecdis.3c00246
Matthias Fellner 1 , Annabel Walsh 1 , Stephen Dela Ahator 2 , Nadia Aftab 2 , Ben Sutherland 3 , Eng W Tan 3 , Alexander T Bakker 4 , Nathaniel I Martin 5 , Mario van der Stelt 4 , Christian S Lentz 2
Affiliation
|
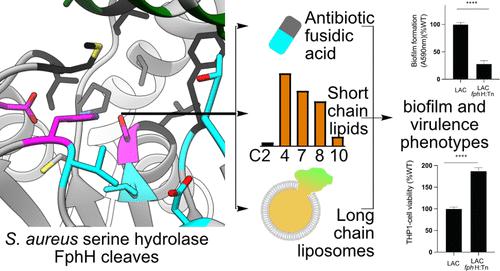
The development of new treatment options for bacterial infections requires access to new targets for antibiotics and antivirulence strategies. Chemoproteomic approaches are powerful tools for profiling and identifying novel druggable target candidates, but their functions often remain uncharacterized. Previously, we used activity-based protein profiling in the opportunistic pathogen Staphylococcus aureus to identify active serine hydrolases termed fluorophosphonate-binding hydrolases (Fph). Here, we provide the first characterization of S. aureus FphH, a conserved, putative carboxylesterase (referred to as yvaK in Bacillus subtilis) at the molecular and cellular level. First, phenotypic characterization of fphH-deficient transposon mutants revealed phenotypes during growth under nutrient deprivation, biofilm formation, and intracellular survival. Biochemical and structural investigations revealed that FphH acts as an esterase and lipase based on a fold well suited to act on a small to long hydrophobic unbranched lipid group within its substrate and can be inhibited by active site-targeting oxadiazoles. Prompted by a previous observation that fphH expression was upregulated in response to fusidic acid, we found that FphH can deacetylate this ribosome-targeting antibiotic, but the lack of FphH function did not infer major changes in antibiotic susceptibility. In conclusion, our results indicate a functional role of this hydrolase in S. aureus stress responses, and hypothetical functions connecting FphH with components of the ribosome rescue system that are conserved in the same gene cluster across Bacillales are discussed. Our atomic characterization of FphH will facilitate the development of specific FphH inhibitors and probes to elucidate its physiological role and validity as a drug target.
中文翻译:

金黄色葡萄球菌中氟膦酸结合水解酶 H (FphH) 功能的生化和细胞表征支持其在细菌应激反应中的作用
细菌感染新治疗方案的开发需要获得抗生素和抗毒策略的新靶点。化学蛋白质组学方法是分析和鉴定新型可成药靶标候选物的强大工具,但其功能通常仍未表征。此前,我们在机会性病原体金黄色葡萄球菌中使用基于活性的蛋白质分析来鉴定称为氟磷酸盐结合水解酶(Fph)的活性丝氨酸水解酶。在这里,我们首次在分子和细胞水平上表征了金黄色葡萄球菌FphH,这是一种保守的、假定的羧酸酯酶(在枯草芽孢杆菌中称为yva K )。首先,fph H 缺陷转座子突变体的表型特征揭示了营养剥夺下生长、生物膜形成和细胞内存活期间的表型。生化和结构研究表明,FphH 作为酯酶和脂肪酶,其折叠非常适合作用于其底物内的小到长的疏水性无支链脂质基团,并且可以被靶向活性位点的恶二唑抑制。先前观察到fph H 表达因夫西地酸而上调,我们发现 FphH 可以使这种核糖体靶向抗生素去乙酰化,但 FphH 功能的缺乏并不意味着抗生素敏感性发生重大变化。总之,我们的结果表明这种水解酶在金黄色葡萄球菌应激反应中的功能作用,并讨论了将 FphH 与核糖体救援系统组件连接起来的假设功能,这些组件在芽孢杆菌目的同一基因簇中保守。我们对 FphH 的原子表征将有助于开发特定的 FphH 抑制剂和探针,以阐明其生理作用和作为药物靶点的有效性。
更新日期:2023-10-12
中文翻译:

金黄色葡萄球菌中氟膦酸结合水解酶 H (FphH) 功能的生化和细胞表征支持其在细菌应激反应中的作用
细菌感染新治疗方案的开发需要获得抗生素和抗毒策略的新靶点。化学蛋白质组学方法是分析和鉴定新型可成药靶标候选物的强大工具,但其功能通常仍未表征。此前,我们在机会性病原体金黄色葡萄球菌中使用基于活性的蛋白质分析来鉴定称为氟磷酸盐结合水解酶(Fph)的活性丝氨酸水解酶。在这里,我们首次在分子和细胞水平上表征了金黄色葡萄球菌FphH,这是一种保守的、假定的羧酸酯酶(在枯草芽孢杆菌中称为yva K )。首先,fph H 缺陷转座子突变体的表型特征揭示了营养剥夺下生长、生物膜形成和细胞内存活期间的表型。生化和结构研究表明,FphH 作为酯酶和脂肪酶,其折叠非常适合作用于其底物内的小到长的疏水性无支链脂质基团,并且可以被靶向活性位点的恶二唑抑制。先前观察到fph H 表达因夫西地酸而上调,我们发现 FphH 可以使这种核糖体靶向抗生素去乙酰化,但 FphH 功能的缺乏并不意味着抗生素敏感性发生重大变化。总之,我们的结果表明这种水解酶在金黄色葡萄球菌应激反应中的功能作用,并讨论了将 FphH 与核糖体救援系统组件连接起来的假设功能,这些组件在芽孢杆菌目的同一基因簇中保守。我们对 FphH 的原子表征将有助于开发特定的 FphH 抑制剂和探针,以阐明其生理作用和作为药物靶点的有效性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号