当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substituted effects on bonding characteristics of cyclopentane-1,3-diyl diradicals monitored by time-resolved infrared spectroscopy
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2023-10-11 , DOI: 10.1002/poc.4575 Masato Kondoh 1 , Shunsuke Kuboki 1 , Hidetaka Kume 1 , Eriku Oda 1 , Manabu Abe 2 , Taka‐aki Ishibashi 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2023-10-11 , DOI: 10.1002/poc.4575 Masato Kondoh 1 , Shunsuke Kuboki 1 , Hidetaka Kume 1 , Eriku Oda 1 , Manabu Abe 2 , Taka‐aki Ishibashi 1
Affiliation
|
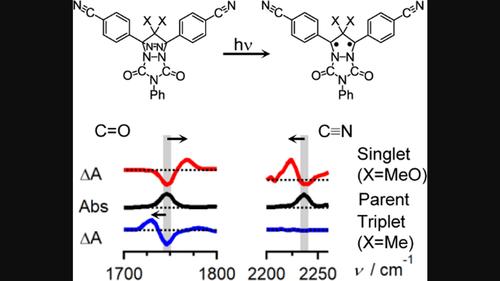
Cyclopentane-1,3-diyl diradicals (DRs) provide excellent opportunities to study the properties of diradicals because their lifetimes can be significantly lengthened to up to milliseconds with the introduction of proper substituents. This study investigated the bonding characteristics of singlet and triplet DRs having C=O and p-cyanophenyl groups (S-DR3 and T-DR3) by monitoring the photo-induced formation of the diradicals from their precursor azo compounds using time-resolved IR (TR-IR) spectroscopy. Upon the formation of S-DR3, a C=O stretching wavenumber was upshifted by 22 cm−1, whereas a C≡N stretching one was downshifted by 12 cm−1. The observed shifts indicate that the unpaired electrons increase and decrease the C=O and C≡N bond orders, respectively. The effects of the unpaired electrons in S-DR3 were similar to those observed in our previous TR-IR studies on a singlet cyclopentane-1,3-diyl diradical having C=O but no C≡N groups (S-DR2) and on that having C≡N but no C=O groups (S-DR1), respectively. Contrastingly, upon the formation of T-DR3, the C=O wavenumber was downshifted by 16 cm−1, indicating that the unpaired electrons decrease the C=O bond order. More notably, no detectable shifts were observed in the C≡N stretching wavenumber. These observations are not clearly explained by a model suggested in the previous studies on S-DRs. Here, we discuss and propose a more elaborated resonance hybrid of DRs that can explain the directions and relative magnitudes of the observed wavenumber shifts irrespective of spin multiplicities. We expect that the findings and suggestions presented here will stimulate research in both organic and theoretical chemistry.
中文翻译:

时间分辨红外光谱监测取代对环戊烷-1,3-二基双自由基键合特性的影响
环戊烷-1,3-二基双自由基(DR)为研究双自由基的性质提供了极好的机会,因为通过引入适当的取代基,它们的寿命可以显着延长至毫秒。本研究通过使用时间分辨红外监测其前体偶氮化合物光诱导形成双自由基,研究了具有 C=O 和对氰基苯基基团(S- DR3和 T- DR3 )的单线态和三线态DR的键合特性(TR-IR)光谱。在S- DR3形成后,C=O伸缩波数上移22cm -1,而C=N伸缩波数下移12cm -1。观察到的变化表明,不成对电子分别增加和减少了 C=O 和 CeqN 键级。S- DR3中不成对电子的影响与我们之前的 TR-IR 研究中观察到的类似,即具有 C=O 但没有 C=N 基团的单线态环戊烷-1,3-二基双自由基 (S- DR2 ) 和分别具有C=N但没有C=O基团(S- DR1)。相反,在T- DR3形成时,C=O波数下移16cm -1,表明不成对电子降低了C=O键级。更值得注意的是,在 C=N 伸缩波数中没有观察到可检测到的位移。这些观察结果并没有通过之前关于 S- DR 的研究中提出的模型得到清楚的解释。在这里,我们讨论并提出了一种更复杂的DR共振混合体,它可以解释观察到的波数变化的方向和相对大小,而与自旋多重性无关。我们期望这里提出的发现和建议将刺激有机化学和理论化学的研究。
更新日期:2023-10-11
中文翻译:

时间分辨红外光谱监测取代对环戊烷-1,3-二基双自由基键合特性的影响
环戊烷-1,3-二基双自由基(DR)为研究双自由基的性质提供了极好的机会,因为通过引入适当的取代基,它们的寿命可以显着延长至毫秒。本研究通过使用时间分辨红外监测其前体偶氮化合物光诱导形成双自由基,研究了具有 C=O 和对氰基苯基基团(S- DR3和 T- DR3 )的单线态和三线态DR的键合特性(TR-IR)光谱。在S- DR3形成后,C=O伸缩波数上移22cm -1,而C=N伸缩波数下移12cm -1。观察到的变化表明,不成对电子分别增加和减少了 C=O 和 CeqN 键级。S- DR3中不成对电子的影响与我们之前的 TR-IR 研究中观察到的类似,即具有 C=O 但没有 C=N 基团的单线态环戊烷-1,3-二基双自由基 (S- DR2 ) 和分别具有C=N但没有C=O基团(S- DR1)。相反,在T- DR3形成时,C=O波数下移16cm -1,表明不成对电子降低了C=O键级。更值得注意的是,在 C=N 伸缩波数中没有观察到可检测到的位移。这些观察结果并没有通过之前关于 S- DR 的研究中提出的模型得到清楚的解释。在这里,我们讨论并提出了一种更复杂的DR共振混合体,它可以解释观察到的波数变化的方向和相对大小,而与自旋多重性无关。我们期望这里提出的发现和建议将刺激有机化学和理论化学的研究。













































 京公网安备 11010802027423号
京公网安备 11010802027423号