当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing insights into the axial coordination effect of M–N4 catalysts on electrocatalytic activity towards the oxygen reduction reaction
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2023-10-11 , DOI: 10.1039/d3ta03701k Youxuan Ni 1 , Weiwei Xie 1 , Jun Chen 1
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2023-10-11 , DOI: 10.1039/d3ta03701k Youxuan Ni 1 , Weiwei Xie 1 , Jun Chen 1
Affiliation
|
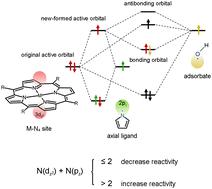
Axial coordination is a powerful strategy to effectively regulate the activity of M–N4 catalysts. However, the underlying mechanism is still not clear. Here, we explore the influence of axial coordination on the activity of Fe/Co/Ni-tetraphenylporphyrin covalent organic frameworks (Fe/Co/Ni-TPP COFs) towards the oxygen reduction reaction (ORR) using the DFT method. It is found that the widely used descriptors (e.g., d-band centers) are not applicable to explain the axial coordination effect on metal reactivity. The reason is that the hybridization of the dz2 orbital of metal centers and pz orbital of axial coordination atoms leads to the formation of new active orbitals, where the effect of the pz orbital should be considered in the design of the activity descriptor. Based on this, an activity descriptor using the total number of electrons in dz2 and pz orbitals is proposed. The results show that the reactivity of the metal center is enhanced by axial coordination if N(dz2) + N(pz) > 2, while it is reduced if N(dz2) + N(pz) ≤ 2. The application of the descriptor is further extended to explain the activity change of Fe/Co/Ni-TPP COFs by different types of axially coordinated atoms. This work provides new insights into the regulation mechanism of the axial coordination effect on metal reactivity.
中文翻译:

揭示 M-N4 催化剂的轴向配位效应对氧还原反应电催化活性的影响
轴向配位是有效调节M–N 4催化剂活性的有力策略。然而,其根本机制仍不清楚。在这里,我们利用 DFT 方法探讨了轴向配位对 Fe/Co/Ni-四苯基卟啉共价有机骨架 (Fe/Co/Ni-TPP COF) 氧还原反应 (ORR) 活性的影响。研究发现广泛使用的描述符(例如d带中心)不适用于解释轴向配位对金属反应性的影响。原因是金属中心的d z 2轨道与轴配位原子的p z轨道杂化导致形成新的活性轨道,在活性描述符的设计中应考虑p z轨道的影响。在此基础上,提出了使用 d z 2和 p z轨道中电子总数的活性描述符。结果表明,当N (d z 2 ) + N (p z ) > 2时,轴向配位会增强金属中心的反应活性,而当N (d z 2 ) + N (p z ) ≤ 2时,会降低金属中心的反应活性。该描述符的应用被进一步扩展,以解释不同类型的轴配位原子对 Fe/Co/Ni-TPP COF 的活性变化。该工作为轴向配位效应对金属反应性的调控机制提供了新的见解。
更新日期:2023-10-11
中文翻译:

揭示 M-N4 催化剂的轴向配位效应对氧还原反应电催化活性的影响
轴向配位是有效调节M–N 4催化剂活性的有力策略。然而,其根本机制仍不清楚。在这里,我们利用 DFT 方法探讨了轴向配位对 Fe/Co/Ni-四苯基卟啉共价有机骨架 (Fe/Co/Ni-TPP COF) 氧还原反应 (ORR) 活性的影响。研究发现广泛使用的描述符(例如d带中心)不适用于解释轴向配位对金属反应性的影响。原因是金属中心的d z 2轨道与轴配位原子的p z轨道杂化导致形成新的活性轨道,在活性描述符的设计中应考虑p z轨道的影响。在此基础上,提出了使用 d z 2和 p z轨道中电子总数的活性描述符。结果表明,当N (d z 2 ) + N (p z ) > 2时,轴向配位会增强金属中心的反应活性,而当N (d z 2 ) + N (p z ) ≤ 2时,会降低金属中心的反应活性。该描述符的应用被进一步扩展,以解释不同类型的轴配位原子对 Fe/Co/Ni-TPP COF 的活性变化。该工作为轴向配位效应对金属反应性的调控机制提供了新的见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号