Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
mRNA Cancer Vaccines: Construction and Boosting Strategies
ACS Nano ( IF 15.8 ) Pub Date : 2023-10-11 , DOI: 10.1021/acsnano.3c05635
Xiaoqing Liu 1, 2, 3 , Pei Huang 2, 3, 4 , Rusen Yang 1 , Hongzhang Deng 2, 3
ACS Nano ( IF 15.8 ) Pub Date : 2023-10-11 , DOI: 10.1021/acsnano.3c05635
Xiaoqing Liu 1, 2, 3 , Pei Huang 2, 3, 4 , Rusen Yang 1 , Hongzhang Deng 2, 3
Affiliation
|
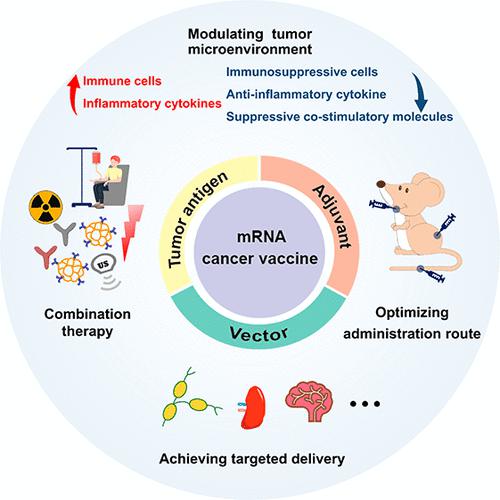
In late 2020, the U.S. Food and Drug Administration (FDA) approved a lipid-based mRNA vaccine for the prevention of COVID-19, which has pushed this field to be more closely studied and motivated researchers to delve deeper into mRNA therapeutics. To date, the research on mRNA cancer vaccines has been developed rapidly, and substantial hopeful therapeutic results have been achieved against various solid tumors in clinical trials. In this review, we first introduce three main components of mRNA cancer vaccines, including mRNA antigens, adjuvants, and delivery vectors. Engineering these components can optimize the therapeutic effects of mRNA cancer vaccines. For instance, appropriate modification of mRNA structure can alleviate the poor stability and innate immunogenicity of mRNA, and the use of mRNA delivery vectors can address the issues of low delivery efficiency in vivo. Second, we emphatically discuss some strategies to further improve the efficacy of mRNA cancer vaccines, namely modulating the immunosuppressive tumor environment, optimizing administration routes, achieving targeting delivery to intended tissues or organs, and employing combination therapy. These strategies can strengthen the tumor inhibitory ability of mRNA cancer vaccines and increase the possibility of tumor elimination. Finally, we point out some challenges in the clinical practice of mRNA cancer vaccines and offer our perspectives on future developments in this rapidly evolving field. It is anticipated that mRNA cancer vaccines will be rapidly developed for clinical cancer therapy in the near future.
中文翻译:

mRNA 癌症疫苗:构建和加强策略
2020年底,美国食品和药物管理局(FDA)批准了一种用于预防COVID-19的脂质mRNA疫苗,这推动了这一领域得到更密切的研究,并激励研究人员更深入地研究mRNA疗法。目前,mRNA癌症疫苗的研究发展迅速,针对多种实体瘤的临床试验已取得了可喜的治疗效果。在这篇综述中,我们首先介绍了mRNA癌症疫苗的三个主要成分,包括mRNA抗原、佐剂和递送载体。对这些组件进行工程设计可以优化 mRNA 癌症疫苗的治疗效果。例如,对mRNA结构进行适当修饰可以缓解mRNA稳定性差和先天免疫原性的问题,而使用mRNA递送载体可以解决体内递送效率低的问题。其次,我们重点讨论了进一步提高mRNA癌症疫苗功效的一些策略,即调节免疫抑制肿瘤环境、优化给药途径、实现靶向递送至预期组织或器官以及采用联合治疗。这些策略可以增强mRNA癌症疫苗的肿瘤抑制能力,增加消除肿瘤的可能性。最后,我们指出了 mRNA 癌症疫苗临床实践中的一些挑战,并对这个快速发展的领域的未来发展提出了我们的看法。预计在不久的将来,mRNA癌症疫苗将迅速开发用于临床癌症治疗。
更新日期:2023-10-11
中文翻译:

mRNA 癌症疫苗:构建和加强策略
2020年底,美国食品和药物管理局(FDA)批准了一种用于预防COVID-19的脂质mRNA疫苗,这推动了这一领域得到更密切的研究,并激励研究人员更深入地研究mRNA疗法。目前,mRNA癌症疫苗的研究发展迅速,针对多种实体瘤的临床试验已取得了可喜的治疗效果。在这篇综述中,我们首先介绍了mRNA癌症疫苗的三个主要成分,包括mRNA抗原、佐剂和递送载体。对这些组件进行工程设计可以优化 mRNA 癌症疫苗的治疗效果。例如,对mRNA结构进行适当修饰可以缓解mRNA稳定性差和先天免疫原性的问题,而使用mRNA递送载体可以解决体内递送效率低的问题。其次,我们重点讨论了进一步提高mRNA癌症疫苗功效的一些策略,即调节免疫抑制肿瘤环境、优化给药途径、实现靶向递送至预期组织或器官以及采用联合治疗。这些策略可以增强mRNA癌症疫苗的肿瘤抑制能力,增加消除肿瘤的可能性。最后,我们指出了 mRNA 癌症疫苗临床实践中的一些挑战,并对这个快速发展的领域的未来发展提出了我们的看法。预计在不久的将来,mRNA癌症疫苗将迅速开发用于临床癌症治疗。

































 京公网安备 11010802027423号
京公网安备 11010802027423号