背景
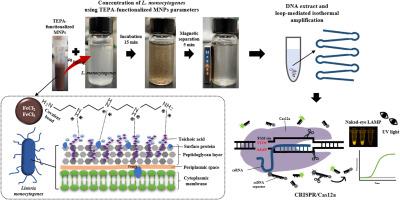
单核细胞增生李斯特菌是一种致病细菌,可导致严重疾病,尤其是在弱势群体中。因此,开发快速、灵敏的检测方法对于预防和管理食源性疾病至关重要。在这项研究中,我们使用四乙烯五胺(TEPA) 功能化磁性纳米颗粒 (MNP) 和基于环介导等温扩增 (LAMP) 的 CRISPR/Cas12a生物传感器分别浓缩和检测单核细胞增生李斯特菌。 LAMP 能够在恒温下扩增 DNA,为即时检测 (POCT) 提供了一种非常合适的方法。 CRISPR/Cas12a 切割 ssDNA 报告基因的能力,加上 TEPA 功能化的 MNP 与带负电荷的细菌的有效附着,形成了一种有前途的生物传感器。
结果
LAMP 检测是通过选择特定引物并设计针对单核细胞增生李斯特菌hly基因内特定区域的 crRNA 序列而精心开发的。我们通过系统探索 59 °C 至 69 °C 的温度范围来选择引物并完善扩增条件,确保获得最佳性能。 LAMP-CRISPR/Cas12a 系统参数的系统优化补充了这一过程。特别是,我们成功地确定了最佳 ssDNA 报告浓度(0-1.2 μM)和 Cas12a 介导的反式切割时间(0-20 分钟),这是支撑基于 LAMP-CRISPR/Cas12a 的生物传感器有效性的关键组成部分。为了优化使用 TEPA 功能化 MNP 捕获单核细胞增多性李斯特菌的参数,通过调整 TEPA 功能化 MNP 浓度、孵育时间和磁分离持续时间,捕获效率显着提高。大体积 (20 mL) 磁分离的灵敏度比传统方法提高了 10 倍。利用TEPA功能化的MNP,基于LAMP-CRISPR/Cas12a的生物传感器在纯培养物中实现了10 0 CFU mL -1的检测限,在金针菇中实现了10 0 CFU g -1的检测限。
意义
这种新技术与基于 LAMP-CRISPR/Cas12a 的生物传感器的集成提高了食品中单核细胞增生李斯特菌检测的准确性和灵敏度,并且它可以成为一种有前途的 POCT 生物传感器。与传统方法相比,灵敏度提高了 10 倍,使得该方法在食品安全和公共卫生领域的病原菌检测方面取得了突破性进展。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Enhanced detection of Listeria monocytogenes using tetraethylenepentamine-functionalized magnetic nanoparticles and LAMP-CRISPR/Cas12a-based biosensor
Background
Listeria monocytogenes is a pathogenic bacterium that can lead to severe illnesses, especially among vulnerable populations. Therefore, the development of rapid and sensitive detection methods is vital to prevent and manage foodborne diseases. In this study, we used tetraethylenepentamine (TEPA)-functionalized magnetic nanoparticles (MNPs) and a loop-mediated isothermal amplification (LAMP)-based CRISPR/Cas12a-based biosensor to concentrate and detect, respectively, L. monocytogenes. LAMP enables DNA amplification at a constant temperature, providing a highly suitable approach for point-of-care testing (POCT). The ability of CRISPR/Cas12a to cleave ssDNA reporter, coupled with TEPA-functionalized MNPs effective attachment to negatively charged bacteria, forms a promising biosensor.
Results
The LAMP assay was meticulously developed by selecting specific primers and designing crRNA sequences targeting a specific region within the hly gene of L. monocytogenes. We selected primer and refined the amplification conditions by systematically exploring a temperature range from 59 °C to 69 °C, ensuring the attainment of optimal performance. This process was complemented by systematic optimization of LAMP-CRISPR/Cas12a system parameters. In particular, we successfully established the optimal ssDNA reporter concentrations (0–1.2 μM) and Cas12a-mediated trans-cleavage times (0–20 min), crucial components that underpin the effectiveness of the LAMP-CRISPR/Cas12a-based biosensor. For optimizing parameters in capturing L. monocytogenes using TEPA-functionalized MNPs, capture efficiency was significantly enhanced through adjustments in TEPA-functionalized MNPs concentration, incubation times, and magnetic separation duration. Large-volume (20 mL) magnetic separation exhibited a 10-fold sensitivity improvement over conventional methods. Utilizing TEPA-functionalized MNPs, the LAMP-CRISPR/Cas12a-based biosensor achieved detection limits of 100 CFU mL−1 in pure cultures and 100 CFU g−1 in enoki mushrooms.
Significance
The integration of this novel technique with the LAMP-CRISPR/Cas12a-based biosensor enhances the accuracy and sensitivity of L. monocytogenes detection in foods, and it can be a promising biosensor for POCT. The 10-fold increase in sensitivity compared to conventional methods makes this approach a groundbreaking advancement in pathogenic bacteria detection for food safety and public health.


































 京公网安备 11010802027423号
京公网安备 11010802027423号