当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stable S-Adenosylmethionine Analogue for Enzymatic Fluoromethylation
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-10-11 , DOI: 10.1021/acscatal.3c03313
Wenrui Wang 1 , Huimin Zhao 1 , Nanhai Yu 1 , Fan Chen 1 , Min Dong 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-10-11 , DOI: 10.1021/acscatal.3c03313
Wenrui Wang 1 , Huimin Zhao 1 , Nanhai Yu 1 , Fan Chen 1 , Min Dong 1
Affiliation
|
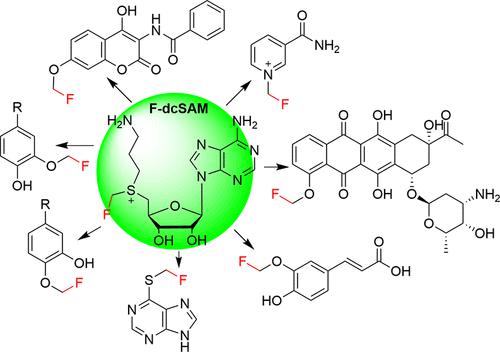
Fluorine is an important atom in medicinal chemistry and agrochemistry, and the fluoromethyl group, an isostere for various functional groups, can improve the metabolic stability and biological activity of compounds. However, enzymes that introduce fluorine and fluorine-containing groups are rare, and performing selective fluoromethylation remains a great challenge in organic chemistry. Biocatalytic fluoromethylation is severely limited by the instability of fluoro S-adenosylmethionine (SAM). Here, we designed and synthesized a stable fluoro SAM analogue, fluoro decarboxyl SAM (F-dcSAM). The F-dcSAM analogue is stable and can be accepted by many O-, S-, and N-methyltransferases, transferring fluoromethyl groups to their substrate. F-dcSAM and methyltransferases were applied to fluoromethylate various compounds, including several bioactive natural products, with high chemo- and regioselectivity. Kinetics studies showed that compared to SAM, F-dcSAM is an analogous or even better substrate for the methyltransferases NtCOMT and DnrK. We further showed that F-dcSAM can be readily prepared enzymatically by halide methyltransferase (HMT) from decarboxyl S-adenosyl-l-homocysteine (dcSAH) and CH2FI. The enzyme cascade reaction involving HMT and methyltransferases can transfer the CH2F group from CH2FI to substrates efficiently with multiple turnovers. Therefore, F-dcSAM can be directly used for enzymatic fluoromethylation or generated in situ through the coupled activities of HMT and methyltransferases. Our results suggest that F-dcSAM is a general abiological cofactor of methyltransferases for late-stage enzymatic fluoromethylation and facilitates the preparation of fluoro analogues of drug molecules. In addition, F-dcSAM is a stable nonaromatic sulfonium ion compound that serves as a fluoromethyl donor, which provides new opportunities for the development of novel CH2F reagents.
中文翻译:

用于酶促氟甲基化的稳定 S-腺苷甲硫氨酸类似物
氟是药物化学和农业化学中的重要原子,氟甲基是多种官能团的电子等排体,可以提高化合物的代谢稳定性和生物活性。然而,引入氟和含氟基团的酶很少见,进行选择性氟甲基化仍然是有机化学中的巨大挑战。氟S-腺苷甲硫氨酸(SAM)的不稳定性严重限制了生物催化氟甲基化。在这里,我们设计并合成了一种稳定的氟SAM类似物,氟脱羧基SAM(F-dcSAM)。F-dcSAM 类似物很稳定,可以被许多O -、S - 和N - 甲基转移酶接受,将氟甲基基团转移到其底物上。F-dcSAM 和甲基转移酶被应用于氟甲基化各种化合物,包括几种具有高化学和区域选择性的生物活性天然产物。动力学研究表明,与 SAM 相比,F-dcSAM 是甲基转移酶Nt COMT 和 DnrK的类似甚至更好的底物。我们进一步表明,F-dcSAM可以通过卤化物甲基转移酶(HMT)从脱羧基S-腺苷-l-高半胱氨酸(dcSAH)和CH 2 FI轻松酶法制备。HMT和甲基转移酶参与的酶级联反应可以将CH 2 F基团从CH 2 FI高效地转移到底物上,并进行多次周转。因此,F-dcSAM可直接用于酶促氟甲基化,或通过HMT和甲基转移酶的耦合活性原位生成。我们的结果表明,F-dcSAM 是用于后期酶促氟甲基化的甲基转移酶的通用非生物辅因子,并有助于药物分子氟类似物的制备。此外,F-dcSAM是一种稳定的非芳香族锍离子化合物,可作为氟甲基供体,为新型CH 2 F试剂的开发提供了新的机遇。
更新日期:2023-10-11
中文翻译:

用于酶促氟甲基化的稳定 S-腺苷甲硫氨酸类似物
氟是药物化学和农业化学中的重要原子,氟甲基是多种官能团的电子等排体,可以提高化合物的代谢稳定性和生物活性。然而,引入氟和含氟基团的酶很少见,进行选择性氟甲基化仍然是有机化学中的巨大挑战。氟S-腺苷甲硫氨酸(SAM)的不稳定性严重限制了生物催化氟甲基化。在这里,我们设计并合成了一种稳定的氟SAM类似物,氟脱羧基SAM(F-dcSAM)。F-dcSAM 类似物很稳定,可以被许多O -、S - 和N - 甲基转移酶接受,将氟甲基基团转移到其底物上。F-dcSAM 和甲基转移酶被应用于氟甲基化各种化合物,包括几种具有高化学和区域选择性的生物活性天然产物。动力学研究表明,与 SAM 相比,F-dcSAM 是甲基转移酶Nt COMT 和 DnrK的类似甚至更好的底物。我们进一步表明,F-dcSAM可以通过卤化物甲基转移酶(HMT)从脱羧基S-腺苷-l-高半胱氨酸(dcSAH)和CH 2 FI轻松酶法制备。HMT和甲基转移酶参与的酶级联反应可以将CH 2 F基团从CH 2 FI高效地转移到底物上,并进行多次周转。因此,F-dcSAM可直接用于酶促氟甲基化,或通过HMT和甲基转移酶的耦合活性原位生成。我们的结果表明,F-dcSAM 是用于后期酶促氟甲基化的甲基转移酶的通用非生物辅因子,并有助于药物分子氟类似物的制备。此外,F-dcSAM是一种稳定的非芳香族锍离子化合物,可作为氟甲基供体,为新型CH 2 F试剂的开发提供了新的机遇。

































 京公网安备 11010802027423号
京公网安备 11010802027423号