当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Formation of 2,3,5-Trisubstituted Furans from 1,3-Dicarbonyls and Hydroxyketones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2023-10-09 , DOI: 10.1002/adsc.202300860 Wanying Yan 1 , Jiaru Shou 1 , Wenyi Qin 1 , Jiayu Mo 2 , Huawen Huang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2023-10-09 , DOI: 10.1002/adsc.202300860 Wanying Yan 1 , Jiaru Shou 1 , Wenyi Qin 1 , Jiayu Mo 2 , Huawen Huang 1
Affiliation
|
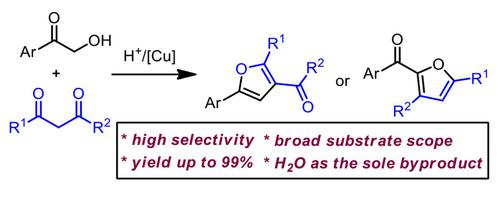
Chemoselective cyclizations of 1,3-dicarbonyl compounds with hydroxyketones for the selective formation of two 2,3,5-trisubstituted furans have been reported. While TsOH-mediated cyclization in DCM afforded 2-acylfurans, the additional copper catalyst in acetone turned over the selectivity for the generation of 3-acylfurans. The featured advantages of both reactions include simple conditions, high yielding, broad substrate scope, gram scalability, and H2O as the sole byproduct.
中文翻译:

由 1,3-二羰基和羟基酮选择性形成 2,3,5-三取代呋喃
已经报道了 1,3-二羰基化合物与羟基酮的化学选择性环化,以选择性形成两个 2,3,5-三取代呋喃。虽然 DCM 中 TsOH 介导的环化反应生成 2-酰基呋喃,但丙酮中额外的铜催化剂却改变了 3-酰基呋喃生成的选择性。两种反应均具有条件简单、收率高、底物范围广、克数可扩展、副产物为H 2 O等特点。
更新日期:2023-10-09
中文翻译:

由 1,3-二羰基和羟基酮选择性形成 2,3,5-三取代呋喃
已经报道了 1,3-二羰基化合物与羟基酮的化学选择性环化,以选择性形成两个 2,3,5-三取代呋喃。虽然 DCM 中 TsOH 介导的环化反应生成 2-酰基呋喃,但丙酮中额外的铜催化剂却改变了 3-酰基呋喃生成的选择性。两种反应均具有条件简单、收率高、底物范围广、克数可扩展、副产物为H 2 O等特点。































 京公网安备 11010802027423号
京公网安备 11010802027423号