Synlett ( IF 1.7 ) Pub Date : 2023-10-09 , DOI: 10.1055/a-2161-9689 Chie Ogasa 1 , Kimika Kayano 1 , Kosuke Namba 2
|
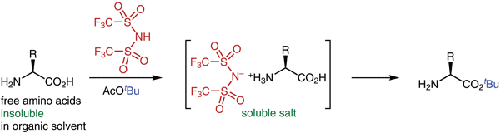
A simple and safe tert-butylation reaction was developed. Treatment of various free amino acids with 1.1 equivalents of bis(trifluoromethanesulfonyl)imide in tert-butyl acetate directly afforded tert-butyl esters with free amino groups quickly and in good yields. In addition, various carboxylic acids and alcohols without amino groups were converted into tert-butyl esters and ethers, respectively, in high yields in the presence of small catalytic amounts of bis(trifluoromethanesulfonyl)imide. All tert-butylation reactions of free amino acids, carboxylic acids, and alcohols proceeded much faster and in higher yields compared with conventional methods.
中文翻译:

羧酸和醇的简单而有效的叔丁基化
开发了一种简单且安全的叔丁基化反应。用1.1当量的双(三氟甲磺酰基)亚胺的乙酸叔丁酯溶液处理各种游离氨基酸,可以快速且高收率地直接得到具有游离氨基的叔丁酯。此外,在少量催化量的双(三氟甲磺酰基)亚胺存在下,各种不含氨基的羧酸和醇分别以高产率转化为叔丁基酯和醚。与传统方法相比,游离氨基酸、羧酸和醇的所有叔丁基化反应进行得更快并且产率更高。






























 京公网安备 11010802027423号
京公网安备 11010802027423号