当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Reducibility of CeO2 Doped with Trivalent Cations
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-10-07 00:00:00 , DOI: 10.1021/acs.jpcc.6b08118 Aoife K. Lucid 1 , Patrick R. L. Keating 1 , Jeremy P. Allen 1 , Graeme W. Watson 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-10-07 00:00:00 , DOI: 10.1021/acs.jpcc.6b08118 Aoife K. Lucid 1 , Patrick R. L. Keating 1 , Jeremy P. Allen 1 , Graeme W. Watson 1
Affiliation
|
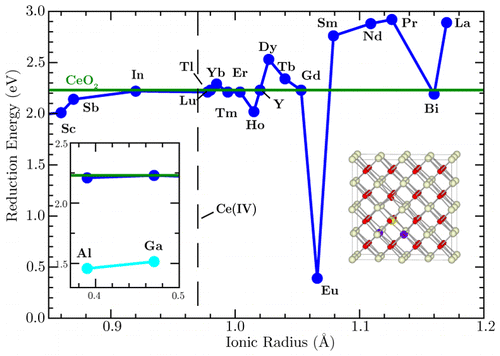
The doping of CeO2 with trivalent cations is a common technique for enhancing ionic conductivity in electrolytes for solid oxide fuel cell applications. However, the local defect structure in these materials is yet to be fully explored. Furthermore, many studies have overlooked the effect of the dopants on the reducibility of CeO2, which is important as electronic conductivity can short-circuit the fuel cell. Density functional theory (DFT)+U calculations have been performed on a series of CeO2 systems doped with trivalent cations. The most stable configuration and the relative attraction between dopant cations and oxygen vacancies were determined, and it was found that the defect structure is principally dependent on the ionic radius of the dopant cations. The reduction energy was found to be dependent on the structure around the dopants but did not vary significantly between dopants of similar ionic radii. From these results, it is possible to suggest which trivalent cations would be most suitable to enhance ionic conductivity without increasing electronic conductivity in solid oxide fuel cell electrolytes.
中文翻译:

三价阳离子掺杂的CeO 2的结构与还原性
用三价阳离子掺杂CeO 2是提高固体氧化物燃料电池应用电解质中离子电导率的常用技术。然而,这些材料中的局部缺陷结构还有待充分研究。此外,许多研究都忽略了掺杂剂对CeO 2还原性的影响,这很重要,因为电子电导率可以使燃料电池短路。对一系列CeO 2进行了密度泛函理论(DFT)+ U计算掺杂三价阳离子的体系。确定了最稳定的构型和掺杂阳离子与氧空位之间的相对吸引力,发现缺陷结构主要取决于掺杂阳离子的离子半径。发现还原能取决于掺杂剂周围的结构,但是在相似离子半径的掺杂剂之间没有显着变化。根据这些结果,可以暗示在不增加固体氧化物燃料电池电解质中的电子电导率的情况下,哪种三价阳离子最适合于增强离子电导率。
更新日期:2016-10-07
中文翻译:

三价阳离子掺杂的CeO 2的结构与还原性
用三价阳离子掺杂CeO 2是提高固体氧化物燃料电池应用电解质中离子电导率的常用技术。然而,这些材料中的局部缺陷结构还有待充分研究。此外,许多研究都忽略了掺杂剂对CeO 2还原性的影响,这很重要,因为电子电导率可以使燃料电池短路。对一系列CeO 2进行了密度泛函理论(DFT)+ U计算掺杂三价阳离子的体系。确定了最稳定的构型和掺杂阳离子与氧空位之间的相对吸引力,发现缺陷结构主要取决于掺杂阳离子的离子半径。发现还原能取决于掺杂剂周围的结构,但是在相似离子半径的掺杂剂之间没有显着变化。根据这些结果,可以暗示在不增加固体氧化物燃料电池电解质中的电子电导率的情况下,哪种三价阳离子最适合于增强离子电导率。































 京公网安备 11010802027423号
京公网安备 11010802027423号