当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphate Binding in PNP Alters Transition-State Analogue Affinity and Subunit Cooperativity
Biochemistry ( IF 2.9 ) Pub Date : 2023-10-09 , DOI: 10.1021/acs.biochem.3c00264 Yacoba V T Minnow 1 , Vern L Schramm 1 , Steven C Almo 1 , Agnidipta Ghosh 1
Biochemistry ( IF 2.9 ) Pub Date : 2023-10-09 , DOI: 10.1021/acs.biochem.3c00264 Yacoba V T Minnow 1 , Vern L Schramm 1 , Steven C Almo 1 , Agnidipta Ghosh 1
Affiliation
|
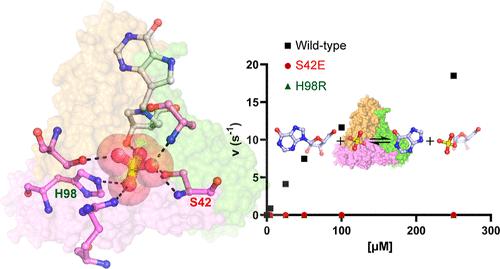
Purine nucleoside phosphorylases (PNPs) catalyze the phosphorolysis of 6-oxypurine nucleosides with an HPO42– dianion nucleophile. Nucleosides and phosphate occupy distinct pockets in the PNP active site. Evaluation of the HPO42– site by mutagenesis, cooperative binding studies, and thermodynamic and structural analysis demonstrate that alterations in the HPO42– binding site can render PNP inactive and significantly impact subunit cooperativity and binding to transition-state analogue inhibitors. Cooperative interactions between the cationic transition-state analogue and the anionic HPO42– nucleophile demonstrate the importance of reforming the transition-state ensemble for optimal inhibition with transition-state analogues. Altered phosphate binding in the catalytic site mutants helps to explain one of the known lethal PNP deficiency syndromes in humans.
中文翻译:

PNP 中的磷酸盐结合改变过渡态类似物亲和力和亚基协同性
嘌呤核苷磷酸化酶 (PNP) 通过 HPO 4 2-二价阴离子亲核试剂催化 6-羟基嘌呤核苷的磷酸解。核苷和磷酸盐占据 PNP 活性位点的不同口袋。通过诱变、协同结合研究以及热力学和结构分析对 HPO 4 2–位点的评估表明,HPO 4 2–结合位点的改变可以使 PNP 失活,并显着影响亚基协同性和与过渡态类似物抑制剂的结合。阳离子过渡态类似物和阴离子 HPO 4 2-亲核试剂之间的协同相互作用证明了重组过渡态整体以实现过渡态类似物最佳抑制的重要性。催化位点突变体中磷酸盐结合的改变有助于解释人类已知的致命性 PNP 缺乏综合征之一。
更新日期:2023-10-09
中文翻译:

PNP 中的磷酸盐结合改变过渡态类似物亲和力和亚基协同性
嘌呤核苷磷酸化酶 (PNP) 通过 HPO 4 2-二价阴离子亲核试剂催化 6-羟基嘌呤核苷的磷酸解。核苷和磷酸盐占据 PNP 活性位点的不同口袋。通过诱变、协同结合研究以及热力学和结构分析对 HPO 4 2–位点的评估表明,HPO 4 2–结合位点的改变可以使 PNP 失活,并显着影响亚基协同性和与过渡态类似物抑制剂的结合。阳离子过渡态类似物和阴离子 HPO 4 2-亲核试剂之间的协同相互作用证明了重组过渡态整体以实现过渡态类似物最佳抑制的重要性。催化位点突变体中磷酸盐结合的改变有助于解释人类已知的致命性 PNP 缺乏综合征之一。































 京公网安备 11010802027423号
京公网安备 11010802027423号