当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diarylborinic Acid-Catalyzed Ring Opening of cis-4-Hydroxymethyl-1,2-Cyclopentene Oxides: Synthesis of 1,2,4-Trisubstituted Cyclopentanes
Organic Letters ( IF 4.9 ) Pub Date : 2023-10-09 , DOI: 10.1021/acs.orglett.3c02886 Shan-Shan Xun 1 , Han Wang 2 , Chang-Bin Yu 2 , Sheng-Mei Lu 2 , Yong-Gui Zhou 2
Organic Letters ( IF 4.9 ) Pub Date : 2023-10-09 , DOI: 10.1021/acs.orglett.3c02886 Shan-Shan Xun 1 , Han Wang 2 , Chang-Bin Yu 2 , Sheng-Mei Lu 2 , Yong-Gui Zhou 2
Affiliation
|
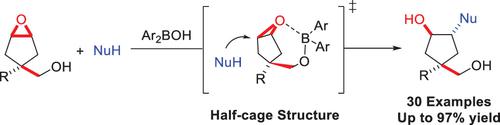
A diarylborinic acid-catalyzed ring opening of cis-4-hydroxymethyl-1,2-cyclopentene oxides was developed with N-nucleophiles including anilines, benzotriazole, and alkylamines, as well as S-nucleophiles, affording 1,2,4-trisubstituted cyclopentane compounds containing a quaternary carbon center. The mechanism study indicated that the “half-cage” structure formed by the epoxide substrate and the catalyst prevents the nucleophiles from attacking the inner side of the “half-cage”, resulting in the desired ring-opening product.
中文翻译:

二芳基硼酸催化顺式-4-羟甲基-1,2-环戊烯氧化物的开环:1,2,4-三取代环戊烷的合成
使用苯胺、苯并三唑和烷基胺等N亲核试剂以及S亲核试剂开发了二芳基硼酸催化的顺式-4-羟甲基-1,2-环戊烯氧化物开环,得到 1,2,4-三取代环戊烷含有季碳中心的化合物。机理研究表明,环氧化物底物和催化剂形成的“半笼”结构可以防止亲核试剂攻击“半笼”的内侧,从而产生所需的开环产物。
更新日期:2023-10-09
中文翻译:

二芳基硼酸催化顺式-4-羟甲基-1,2-环戊烯氧化物的开环:1,2,4-三取代环戊烷的合成
使用苯胺、苯并三唑和烷基胺等N亲核试剂以及S亲核试剂开发了二芳基硼酸催化的顺式-4-羟甲基-1,2-环戊烯氧化物开环,得到 1,2,4-三取代环戊烷含有季碳中心的化合物。机理研究表明,环氧化物底物和催化剂形成的“半笼”结构可以防止亲核试剂攻击“半笼”的内侧,从而产生所需的开环产物。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号