Nano Research ( IF 9.5 ) Pub Date : 2023-10-09 , DOI: 10.1007/s12274-023-6152-6
Chunling Bo , Li Zhang , Xiaolong Liu , Huaiqiu Chang , Yingxue Sun , Xinyi Zhang , Ting Tan , Lingyu Piao
|
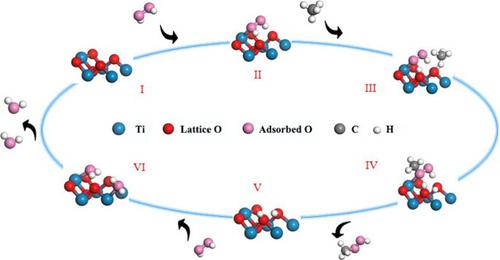
Partial oxidation of methane into primary oxidation products with high value remains a challenge. In this work, photocatalytic oxidation of methane (CH4) with high methyl hydroperoxide (CH3OOH) selectivity is achieved using pure titanium oxide (TiO2) without any cocatalyst at room temperature and atmospheric pressure. The CH3OOH production rate can reach up to 2050 ± 88 µmol·g−1·h−1 at pH ≈ 7.0 with 100% selectivity in the liquid product. The stable reaction cycle can reach more than 30 times. This low-cost system achieves superior CH4 conversion activity and selectivity compared with similar work. The energy of hydrogen peroxide (H2O2) to adsorbed hydroperoxyl radical (⋆OOH) has a significantly lower reaction energy than conversion to adsorbed hydroxyl radical (⋆OH) on the (210) surface of the TiO2. The ⋆OOH preferentially combines with methyl radical (·CH3) to form the most energetically favorable CH3OOH. The mild oxidative environment of this system prevents the reduction of CH3OOH to CH3OH or over-oxidation of CH4, which ensures the final CH3OOH with high selectivity and stability. This work provided a low-cost but highly efficient method to achieve partial oxidation with superior selectivity, i.e., to convert CH4 into high-value chemicals.
中文翻译:

甲烷高选择性光催化氧化成甲基氢过氧化物
将甲烷部分氧化成高价值的初级氧化产物仍然是一个挑战。在这项工作中,在室温和大气压下,使用纯二氧化钛(TiO 2 )在没有任何助催化剂的情况下实现了具有高甲基氢过氧化物(CH 3 OOH)选择性的甲烷(CH 4 )的光催化氧化。在pH ≈ 7.0 时, CH 3 OOH 产率可达2050 ± 88 µmol·g -1 ·h -1,液体产物的选择性为100%。稳定反应循环可达30次以上。与类似工作相比,这种低成本系统实现了卓越的 CH 4转化活性和选择性。过氧化氢(H 2 O 2 )转化为吸附的氢过氧自由基(⋆OOH)的能量比转化为TiO 2 (210)表面上吸附的羟基自由基(⋆OH)的反应能量显着更低。⋆OOH优先与甲基自由基(·CH 3 )结合形成能量上最有利的CH 3 OOH。该系统温和的氧化环境防止了CH 3 OOH还原为CH 3 OH或CH 4的过度氧化,保证了最终CH 3 OOH的高选择性和稳定性。这项工作提供了一种低成本但高效的方法来实现具有优异选择性的部分氧化,即将CH 4转化为高价值化学品。

































 京公网安备 11010802027423号
京公网安备 11010802027423号