Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Membrane anchoring of the DIRAS3 N-terminal extension permits tumor suppressor function
iScience ( IF 4.6 ) Pub Date : 2023-10-06 , DOI: 10.1016/j.isci.2023.108151
Xiaowen Liang 1 , Sung Yun Jung 2 , Lon Wolf Fong 1 , Gamze Bildik 1 , Joshua P Gray 1 , Weiqun Mao 1 , Shuxing Zhang 1 , Steven W Millward 3 , Alemayehu A Gorfe 4 , Yong Zhou 4 , Zhen Lu 1 , Robert C Bast 1
iScience ( IF 4.6 ) Pub Date : 2023-10-06 , DOI: 10.1016/j.isci.2023.108151
Xiaowen Liang 1 , Sung Yun Jung 2 , Lon Wolf Fong 1 , Gamze Bildik 1 , Joshua P Gray 1 , Weiqun Mao 1 , Shuxing Zhang 1 , Steven W Millward 3 , Alemayehu A Gorfe 4 , Yong Zhou 4 , Zhen Lu 1 , Robert C Bast 1
Affiliation
|
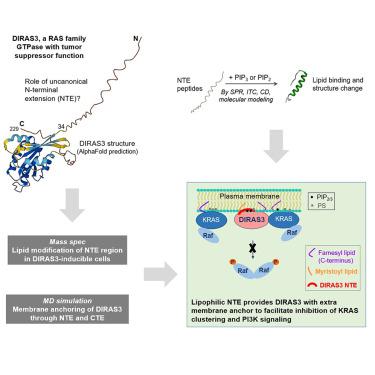
is an imprinted tumor suppressor gene encoding a GTPase that has a distinctive N-terminal extension (NTE) not found in other RAS proteins. This NTE and the prenylated C-terminus are required for DIRAS3-mediated inhibition of RAS/MAP signaling and PI3K activity at the plasma membrane. In this study, we applied biochemical, biophysical, and computational methods to characterize the structure and function of the NTE. The NTE peptide recognizes phosphoinositides PI(3,4,5)P and PI(4,5)P with rapid kinetics and strong affinity. Lipid binding induces NTE structural change from disorder to amphipathic helix. Mass spectrometry identified N-myristoylation of DIRAS3. All-atom molecular dynamic simulations predict DIRAS3 could adhere to the membrane through both termini, suggesting the NTE is involved in targeting and stabilizing DIRAS3 on the membrane by double anchoring. Overall, our results are consistent with DIRAS3’s function as a tumor suppressor, whereby the membrane-bound DIRAS3 can effectively target PI3K and KRAS at the membrane.
中文翻译:

DIRAS3 N 端延伸的膜锚定可实现肿瘤抑制功能
是一种印记肿瘤抑制基因,编码 GTP 酶,具有其他 RAS 蛋白中未发现的独特 N 末端延伸 (NTE)。该 NTE 和异戊二烯化的 C 末端是 DIRAS3 介导的对质膜上 RAS/MAP 信号传导和 PI3K 活性的抑制所必需的。在这项研究中,我们应用生物化学、生物物理和计算方法来表征 NTE 的结构和功能。 NTE 肽以快速动力学和强亲和力识别磷酸肌醇 PI(3,4,5)P 和 PI(4,5)P。脂质结合诱导 NTE 结构从无序变为两亲螺旋。质谱分析鉴定了 DIRAS3 的 N-肉豆蔻酰化。全原子分子动力学模拟预测 DIRAS3 可以通过两个末端粘附在膜上,这表明 NTE 参与通过双锚定将 DIRAS3 靶向和稳定在膜上。总体而言,我们的结果与 DIRAS3 作为肿瘤抑制因子的功能一致,即膜结合的 DIRAS3 可以有效地靶向膜上的 PI3K 和 KRAS。
更新日期:2023-10-06
中文翻译:

DIRAS3 N 端延伸的膜锚定可实现肿瘤抑制功能
是一种印记肿瘤抑制基因,编码 GTP 酶,具有其他 RAS 蛋白中未发现的独特 N 末端延伸 (NTE)。该 NTE 和异戊二烯化的 C 末端是 DIRAS3 介导的对质膜上 RAS/MAP 信号传导和 PI3K 活性的抑制所必需的。在这项研究中,我们应用生物化学、生物物理和计算方法来表征 NTE 的结构和功能。 NTE 肽以快速动力学和强亲和力识别磷酸肌醇 PI(3,4,5)P 和 PI(4,5)P。脂质结合诱导 NTE 结构从无序变为两亲螺旋。质谱分析鉴定了 DIRAS3 的 N-肉豆蔻酰化。全原子分子动力学模拟预测 DIRAS3 可以通过两个末端粘附在膜上,这表明 NTE 参与通过双锚定将 DIRAS3 靶向和稳定在膜上。总体而言,我们的结果与 DIRAS3 作为肿瘤抑制因子的功能一致,即膜结合的 DIRAS3 可以有效地靶向膜上的 PI3K 和 KRAS。

































 京公网安备 11010802027423号
京公网安备 11010802027423号