当前位置:
X-MOL 学术
›
Appl. Catal. B Environ. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular elucidation of CO2 methanation over a highly active, selective and stable LaNiO3/CeO2-derived catalyst by in situ FTIR and NAP-XPS
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2023-10-07 , DOI: 10.1016/j.apcatb.2023.123367 Jon A. Onrubia-Calvo , Sergio López-Rodríguez , Ignacio J. Villar-García , Virginia Pérez-Dieste , Agustín Bueno-López , Juan R. González-Velasco
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2023-10-07 , DOI: 10.1016/j.apcatb.2023.123367 Jon A. Onrubia-Calvo , Sergio López-Rodríguez , Ignacio J. Villar-García , Virginia Pérez-Dieste , Agustín Bueno-López , Juan R. González-Velasco
|
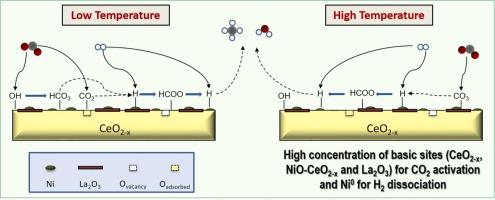
The CO methanation mechanism over the highly active (75.1 h), selective (>92%) and stable 10% LaNiO/CeO-derived catalyst is still unresolved. The surface of the catalyst is monitored under hydrogenation (H), oxidizing (CO) and CO methanation (H +CO) conditions by near ambient pressure X-ray photoelectron spectroscopy (NAP-XPS) using synchrotron radiation. Meanwhile, the main reaction intermediates are identified by FTIR analysis. NAP-XPS experiments confirm that LaNiO perovskite reduction leads to the ex-solution of Ni nanoparticles and NiCeO and NiLaO interfaces conformation, favouring the CO adsorption and the H dissociation/transfer. FTIR experiments combined with the C1s spectra (NAP-XPS) suggest that the CO activation occurs on CeO (oxygen vacancies and OH) at low temperatures, in the form of bicarbonates; whereas, mono-/bidentate carbonates are formed on different strength LaO sites at increasing temperatures. These species are consecutively reduced to formates, as the main reaction intermediate, and methane by the H spilled from Ni nanoparticles near to NiOCeO and NiOLaO interfaces.
中文翻译:

通过原位 FTIR 和 NAP-XPS 对高活性、选择性和稳定的 LaNiO3/CeO2 衍生催化剂上的 CO2 甲烷化进行分子阐明
高活性(75.1 h)、选择性(>92%)和稳定的 10% LaNiO/CeO 衍生催化剂的 CO 甲烷化机理仍未解决。通过使用同步加速器辐射的近环境压力 X 射线光电子能谱 (NAP-XPS) 在氢化 (H)、氧化 (CO) 和 CO 甲烷化 (H +CO) 条件下监测催化剂的表面。同时,通过FTIR分析鉴定了主要反应中间体。 NAP-XPS实验证实LaNiO钙钛矿还原导致Ni纳米粒子和NiCeO和NiLaO界面构象的脱溶,有利于CO吸附和H离解/转移。 FTIR 实验与 C1s 光谱 (NAP-XPS) 相结合表明,CO 活化在低温下以碳酸氢盐的形式发生在 CeO(氧空位和 OH)上;然而,随着温度的升高,单齿/双齿碳酸盐会在不同强度的 La2O 位点上形成。这些物质被 NiOCeO 和 NiOLaO 界面附近的 Ni 纳米粒子中溢出的 H 连续还原为甲酸盐(作为主要反应中间体)和甲烷。
更新日期:2023-10-07
中文翻译:

通过原位 FTIR 和 NAP-XPS 对高活性、选择性和稳定的 LaNiO3/CeO2 衍生催化剂上的 CO2 甲烷化进行分子阐明
高活性(75.1 h)、选择性(>92%)和稳定的 10% LaNiO/CeO 衍生催化剂的 CO 甲烷化机理仍未解决。通过使用同步加速器辐射的近环境压力 X 射线光电子能谱 (NAP-XPS) 在氢化 (H)、氧化 (CO) 和 CO 甲烷化 (H +CO) 条件下监测催化剂的表面。同时,通过FTIR分析鉴定了主要反应中间体。 NAP-XPS实验证实LaNiO钙钛矿还原导致Ni纳米粒子和NiCeO和NiLaO界面构象的脱溶,有利于CO吸附和H离解/转移。 FTIR 实验与 C1s 光谱 (NAP-XPS) 相结合表明,CO 活化在低温下以碳酸氢盐的形式发生在 CeO(氧空位和 OH)上;然而,随着温度的升高,单齿/双齿碳酸盐会在不同强度的 La2O 位点上形成。这些物质被 NiOCeO 和 NiOLaO 界面附近的 Ni 纳米粒子中溢出的 H 连续还原为甲酸盐(作为主要反应中间体)和甲烷。































 京公网安备 11010802027423号
京公网安备 11010802027423号