当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphonate and Thiasugar Analogues of Glucosamine-6-phosphate: Activation of the glmS Riboswitch and Antibiotic Activity
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2023-10-04 , DOI: 10.1021/acschembio.3c00452 Bjarne Silkenath 1 , Dennis Kläge 1 , Hanna Altwein 1 , Nina Schmidhäuser 1 , Günter Mayer 2 , Jörg S Hartig 1 , Valentin Wittmann 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2023-10-04 , DOI: 10.1021/acschembio.3c00452 Bjarne Silkenath 1 , Dennis Kläge 1 , Hanna Altwein 1 , Nina Schmidhäuser 1 , Günter Mayer 2 , Jörg S Hartig 1 , Valentin Wittmann 1
Affiliation
|
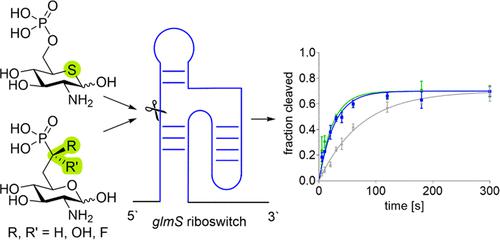
The glmS riboswitch is a motif found in 5′-untranslated regions of bacterial mRNA that controls the synthesis of glucosamine-6-phosphate (GlcN6P), an essential building block for the bacterial cell wall, by a feedback mechanism. Activation of the glmS riboswitch by GlcN6P mimics interferes with the ability of bacteria to synthesize its cell wall. Accordingly, GlcN6P mimics acting as glmS activators are promising candidates for future antibiotic drugs that may overcome emerging bacterial resistance against established antibiotics. We describe the synthesis of a series of phosphonate mimics of GlcN6P as well as the thiasugar analogue of GlcN6P. The phosphonate mimics differ in their pKa value to answer the question of whether derivatives with a pKa matching that of GlcN6P would be efficient glmS activators. We found that all derivatives activate the riboswitch, however, less efficiently than GlcN6P. This observation can be explained by the missing hydrogen bonds in the case of phosphonates and is valuable information for the design of future GlcN6P mimics. The thiasugar analogue of GlcN6P on the other hand turned out to be a glmS riboswitch activator with the same activity as the natural metabolite GlcN6P. The nonphosphorylated thiasugar displayed antimicrobial activity against certain bacilli. Therefore, the compound is a promising lead structure for the development of future antibiotics with a potentially novel mode of action.
中文翻译:

6-磷酸氨基葡萄糖的磷酸盐和硫糖类似物:glmS 核糖开关的激活和抗生素活性
glmS核糖开关是在细菌 mRNA 5' 非翻译区中发现的基序,通过反馈机制控制 6-磷酸葡萄糖胺 (GlcN6P) 的合成, GlcN6P是细菌细胞壁的重要组成部分。GlcN6P 模拟物激活glmS核糖开关会干扰细菌合成细胞壁的能力。因此,作为glmS激活剂的 GlcN6P 模拟物是未来抗生素药物的有前途的候选药物,可以克服新出现的细菌对现有抗生素的耐药性。我们描述了一系列 GlcN6P 的膦酸盐模拟物以及 GlcN6P 的硫糖类似物的合成。磷酸盐模拟物的 p K a值不同,以回答 p K a与GlcN6P 匹配的衍生物是否是有效的glmS激活剂的问题。我们发现所有衍生物激活核糖开关的效率都低于 GlcN6P。这一观察结果可以用膦酸盐中缺失的氢键来解释,并且对于未来 GlcN6P 模拟物的设计来说是有价值的信息。另一方面,GlcN6P 的硫糖类似物被证明是一种glmS核糖开关激活剂,其活性与天然代谢物 GlcN6P 相同。非磷酸化硫糖对某些杆菌表现出抗菌活性。因此,该化合物是未来抗生素开发的一种有前景的先导结构,具有潜在的新颖作用模式。
更新日期:2023-10-04
中文翻译:

6-磷酸氨基葡萄糖的磷酸盐和硫糖类似物:glmS 核糖开关的激活和抗生素活性
glmS核糖开关是在细菌 mRNA 5' 非翻译区中发现的基序,通过反馈机制控制 6-磷酸葡萄糖胺 (GlcN6P) 的合成, GlcN6P是细菌细胞壁的重要组成部分。GlcN6P 模拟物激活glmS核糖开关会干扰细菌合成细胞壁的能力。因此,作为glmS激活剂的 GlcN6P 模拟物是未来抗生素药物的有前途的候选药物,可以克服新出现的细菌对现有抗生素的耐药性。我们描述了一系列 GlcN6P 的膦酸盐模拟物以及 GlcN6P 的硫糖类似物的合成。磷酸盐模拟物的 p K a值不同,以回答 p K a与GlcN6P 匹配的衍生物是否是有效的glmS激活剂的问题。我们发现所有衍生物激活核糖开关的效率都低于 GlcN6P。这一观察结果可以用膦酸盐中缺失的氢键来解释,并且对于未来 GlcN6P 模拟物的设计来说是有价值的信息。另一方面,GlcN6P 的硫糖类似物被证明是一种glmS核糖开关激活剂,其活性与天然代谢物 GlcN6P 相同。非磷酸化硫糖对某些杆菌表现出抗菌活性。因此,该化合物是未来抗生素开发的一种有前景的先导结构,具有潜在的新颖作用模式。































 京公网安备 11010802027423号
京公网安备 11010802027423号