当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring a β-Amino Acid with a Seven-Membered Ring Constraint as a Foldamer Building Block for Nontraditional Helices
Organic Letters ( IF 4.9 ) Pub Date : 2023-10-06 , DOI: 10.1021/acs.orglett.3c02746 Nuri Seo 1 , Hoyang Son 1 , Yonghan Kim 1 , Ilia A Guzei 2 , Philjae Kang 1 , Soo Hyuk Choi 1
Organic Letters ( IF 4.9 ) Pub Date : 2023-10-06 , DOI: 10.1021/acs.orglett.3c02746 Nuri Seo 1 , Hoyang Son 1 , Yonghan Kim 1 , Ilia A Guzei 2 , Philjae Kang 1 , Soo Hyuk Choi 1
Affiliation
|
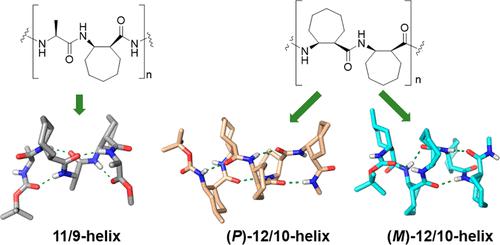
We explored trans- and cis-2-aminocycloheptanecarboxylic acid (ACHpC) as potential building blocks for helical foldamers. trans-ACHpC does not show sufficient folding propensity in unnatural peptides. cis-ACHpC promotes nontraditional helices of two unnatural peptide backbones: the 11/9-helix for 1:1 α/β-peptides and the 12/10-helix for β-peptides with interconvertible handedness. The two opposite-handed 12/10-helices rapidly interconvert in solution by pseudorotation of the two twist chair forms of the cycloheptane moiety in each cis-ACHpC residue.
中文翻译:

探索具有七元环约束的 β-氨基酸作为非传统螺旋的 Foldamer 构建模块
我们探索了反式和顺式-2-氨基环庚烷甲酸(ACHpC)作为螺旋折叠体的潜在构建模块。trans -ACHpC 在非天然肽中没有表现出足够的折叠倾向。cis -ACHpC 促进两种非天然肽主链的非传统螺旋:1:1 α/β-肽的 11/9-螺旋和具有可互换手性的 β-肽的 12/10-螺旋。通过每个顺式-ACHpC 残基中环庚烷部分的两个扭椅形式的假旋转,两个相反旋向的 12/10 螺旋在溶液中快速相互转化。
更新日期:2023-10-06
中文翻译:

探索具有七元环约束的 β-氨基酸作为非传统螺旋的 Foldamer 构建模块
我们探索了反式和顺式-2-氨基环庚烷甲酸(ACHpC)作为螺旋折叠体的潜在构建模块。trans -ACHpC 在非天然肽中没有表现出足够的折叠倾向。cis -ACHpC 促进两种非天然肽主链的非传统螺旋:1:1 α/β-肽的 11/9-螺旋和具有可互换手性的 β-肽的 12/10-螺旋。通过每个顺式-ACHpC 残基中环庚烷部分的两个扭椅形式的假旋转,两个相反旋向的 12/10 螺旋在溶液中快速相互转化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号