当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Tetrahydro-indolones through Rh(III)-Catalyzed [3 + 2] Annulation of Enaminones with Iodonium Ylides
Organic Letters ( IF 4.9 ) Pub Date : 2023-10-03 , DOI: 10.1021/acs.orglett.3c02515
Mingshuai Zhang 1 , Longkun Chen 1 , Haifeng Sun 1 , Zhuoyuan Liu 1 , Jiuzhong Huang 2 , Fuchao Yu 1
Organic Letters ( IF 4.9 ) Pub Date : 2023-10-03 , DOI: 10.1021/acs.orglett.3c02515
Mingshuai Zhang 1 , Longkun Chen 1 , Haifeng Sun 1 , Zhuoyuan Liu 1 , Jiuzhong Huang 2 , Fuchao Yu 1
Affiliation
|
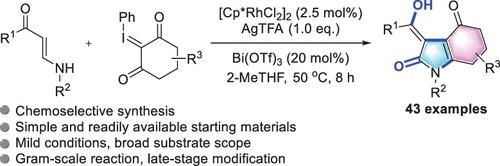
An unprecedented protocol for a Rh(III)-catalyzed [3 + 2] annulation from simple and readily available enaminones and iodonium ylides has been developed. The novel strategy allows for access to a new class of structurally diverse tetrahydro-indolones with high efficiency and a broad substrate scope. In addition, this transformation represents the first example of the selective Rh(III)-catalyzed alkenyl C–H bond functionalization and annulation of enaminones. Finally, the potential applications of this protocol are demonstrated through gram-scale reaction and late-stage modification.
中文翻译:

Rh(III) 催化烯胺酮与碘鎓叶立德成环反应合成四氢吲哚酮
已经开发出一种前所未有的方案,用于从简单易得的烯胺酮和碘鎓叶立德进行 Rh(III) 催化的 [3 + 2] 环化。这种新颖的策略允许获得一类结构多样的新型四氢吲哚酮,具有高效率和广泛的底物范围。此外,这种转化代表了选择性 Rh(III) 催化的烯基 C-H 键功能化和烯胺酮环化的第一个例子。最后,通过克级反应和后期修饰证明了该协议的潜在应用。
更新日期:2023-10-03
中文翻译:

Rh(III) 催化烯胺酮与碘鎓叶立德成环反应合成四氢吲哚酮
已经开发出一种前所未有的方案,用于从简单易得的烯胺酮和碘鎓叶立德进行 Rh(III) 催化的 [3 + 2] 环化。这种新颖的策略允许获得一类结构多样的新型四氢吲哚酮,具有高效率和广泛的底物范围。此外,这种转化代表了选择性 Rh(III) 催化的烯基 C-H 键功能化和烯胺酮环化的第一个例子。最后,通过克级反应和后期修饰证明了该协议的潜在应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号