当前位置:
X-MOL 学术
›
Adv. Mater. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biolistic Delivery of Photosensitizer-Loaded Porous Si Carriers for Localized Photodynamic Therapy
Advanced Materials Technologies ( IF 6.4 ) Pub Date : 2023-10-05 , DOI: 10.1002/admt.202300877 Elina Haimov‐Talmoud 1, 2, 3 , Michal Rosenberg 4 , Sofia Arshavsky‐Graham 4 , Eli Varon 2, 3 , Orit Shefi 2, 3 , Ester Segal 4, 5
Advanced Materials Technologies ( IF 6.4 ) Pub Date : 2023-10-05 , DOI: 10.1002/admt.202300877 Elina Haimov‐Talmoud 1, 2, 3 , Michal Rosenberg 4 , Sofia Arshavsky‐Graham 4 , Eli Varon 2, 3 , Orit Shefi 2, 3 , Ester Segal 4, 5
Affiliation
|
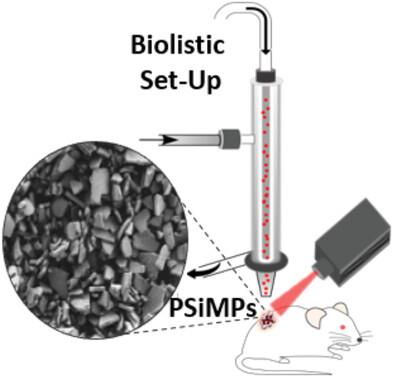
Among numerous approaches for treating cancer, clinically approved photodynamic therapy (PDT) is considered a promising non-invasive therapeutic strategy for solid tumors. While PDT has distinct advantages over conventional cancer treatments, systemic exposure to the photosensitizer and its stability are some of the limitations of clinical PDT. Herein, a therapeutic strategy for highly localized focal PDT is introduced based on direct biolistic delivery of photosensitizer-loaded carriers to cancerous tumors. Degradable porous silicon microparticles (PSiMPs) are used as efficient carriers for the photosensitizer, meso-tetrahydroxy-phenylchlorin (mTHPC), and its conjugates with gold nanoparticles (AuNP-mTHPC conjugates). The loaded PSiMP carriers are successfully bombarded using a pneumatic gene gun to breast cancer cells in vitro and into tumor xenografts in vivo, and subsequent uptake of the released photosensitizer payload is demonstrated. PDT irradiation is commenced after 24 h based on the release profile, resulting in complete cell death in vitro and substantial inhibition of tumor growth in vivo. Notably, using empty PSiMP carriers also leads to some tumor growth inhibition, ascribing to its intrinsic photosensitizing activity. Treatment with AuNP-mTHPC-loaded PSiMPs exhibits a superior therapeutic effect in comparison to bombarded mTHPC-loaded carriers and administration of free mTHPC. This biolistic delivery scheme may be advantageous for precision photodynamic therapy.
中文翻译:

用于局部光动力治疗的负载光敏剂的多孔硅载体的生物射弹递送
在众多治疗癌症的方法中,临床批准的光动力疗法(PDT)被认为是一种有前途的实体瘤非侵入性治疗策略。虽然 PDT 比传统癌症治疗具有明显的优势,但光敏剂的全身暴露及其稳定性是临床 PDT 的一些局限性。在此,引入了一种基于直接生物射弹将负载光敏剂的载体递送至癌性肿瘤的高度局部局部PDT的治疗策略。可降解多孔硅微粒(PSiMPs)被用作光敏剂、内消旋四羟基苯二氯林(mTHPC)及其与金纳米粒子的缀合物(AuNP-mTHPC缀合物)的有效载体。使用气动基因枪成功地将装载的 PSiMP 载体轰击到体外的乳腺癌细胞和体内的肿瘤异种移植物中,并证明了随后对释放的光敏剂有效负载的吸收。根据释放曲线,24 小时后开始 PDT 照射,导致体外细胞完全死亡,并显着抑制体内肿瘤生长。值得注意的是,使用空 PSiMP 载体也会导致一些肿瘤生长抑制,这归因于其固有的光敏活性。与轰击负载 mTHPC 的载体和施用游离 mTHPC 相比,使用负载 AuNP-mTHPC 的 PSiMP 进行治疗表现出更好的治疗效果。这种生物射弹递送方案可能有利于精确光动力治疗。
更新日期:2023-10-05
中文翻译:

用于局部光动力治疗的负载光敏剂的多孔硅载体的生物射弹递送
在众多治疗癌症的方法中,临床批准的光动力疗法(PDT)被认为是一种有前途的实体瘤非侵入性治疗策略。虽然 PDT 比传统癌症治疗具有明显的优势,但光敏剂的全身暴露及其稳定性是临床 PDT 的一些局限性。在此,引入了一种基于直接生物射弹将负载光敏剂的载体递送至癌性肿瘤的高度局部局部PDT的治疗策略。可降解多孔硅微粒(PSiMPs)被用作光敏剂、内消旋四羟基苯二氯林(mTHPC)及其与金纳米粒子的缀合物(AuNP-mTHPC缀合物)的有效载体。使用气动基因枪成功地将装载的 PSiMP 载体轰击到体外的乳腺癌细胞和体内的肿瘤异种移植物中,并证明了随后对释放的光敏剂有效负载的吸收。根据释放曲线,24 小时后开始 PDT 照射,导致体外细胞完全死亡,并显着抑制体内肿瘤生长。值得注意的是,使用空 PSiMP 载体也会导致一些肿瘤生长抑制,这归因于其固有的光敏活性。与轰击负载 mTHPC 的载体和施用游离 mTHPC 相比,使用负载 AuNP-mTHPC 的 PSiMP 进行治疗表现出更好的治疗效果。这种生物射弹递送方案可能有利于精确光动力治疗。































 京公网安备 11010802027423号
京公网安备 11010802027423号