当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactive Extraction of Levulinic Acid from Aqueous Solutions Using Trioctylamine with Diluents 2-Ethyl-1-hexanol, 4-Methylpentan-2-one, and Isoamyl Alcohol
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2023-10-05 , DOI: 10.1021/acs.jced.3c00309 Behnaz Asadzadeh 1 , Mohammed Saad 1 , Petri Uusi-Kyyny 1 , Ville Alopaeus 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2023-10-05 , DOI: 10.1021/acs.jced.3c00309 Behnaz Asadzadeh 1 , Mohammed Saad 1 , Petri Uusi-Kyyny 1 , Ville Alopaeus 1
Affiliation
|
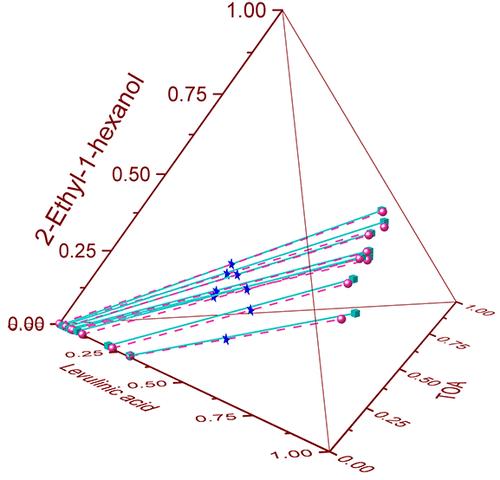
Separating carboxylic acids from aqueous solutions is a challenge, and reactive extraction has been examined as an attractive alternative. This study investigates the reactive extraction of levulinic acid (LA) using trioctylamine (TOA) in various diluents, such as 2-ethyl-1-hexanol, 4-methylpentan-2-one (MIBK), and isoamyl alcohol. For this purpose, liquid–liquid equilibrium (LLE) data was experimentally obtained for the mix of LA + TOA + H2O + diluents at T = 293.15 K and atmospheric pressure. From the obtained data, the ability of various TOA/diluent mixtures was evaluated in terms of distribution coefficient (KD). Isoamyl alcohol was found to be an effective diluent at the diluted region (wLAaq < 0.1) with a KD value of 9.4. However, increasing the concentration of LA resulted in approximately the same extraction ability as the other tested diluents with TOA. Furthermore, the nonrandom two-liquid (NRTL) excess Gibbs energy model was applied to correlate the tie lines. The root-mean-square deviations (RMSD) in liquid mass fraction obtained with the NRTL model for the experimental data of the above-mentioned different LLE systems of 2-ethyl-1-hexanol, MIBK, and isoamyl alcohol were 0.013, 0.018, and 0.016, respectively. Additionally, the KD values of the systems were also computed.
中文翻译:

使用三辛胺和稀释剂 2-乙基-1-己醇、4-甲基戊-2-酮和异戊醇从水溶液中反应萃取乙酰丙酸
从水溶液中分离羧酸是一项挑战,反应萃取已被视为一种有吸引力的替代方案。本研究研究了使用三辛胺 (TOA) 在各种稀释剂(例如 2-乙基-1-己醇、4-甲基戊-2-酮 (MIBK) 和异戊醇)中反应萃取乙酰丙酸 (LA)。为此,在T = 293.15 K 和大气压力下,通过实验获得了 LA + TOA + H 2 O + 稀释剂混合物的液液平衡 (LLE) 数据。根据获得的数据,根据分配系数( K D )评估各种 TOA/稀释剂混合物的能力。发现异戊醇是稀释区域 ( w LA aq < 0.1) 的有效稀释剂, K D值为 9.4。然而,增加 LA 浓度导致的萃取能力与其他测试的 TOA 稀释剂大致相同。此外,应用非随机双液(NRTL)过量吉布斯能量模型来关联联络线。上述2-乙基-1-己醇、MIBK、异戊醇不同LLE体系的实验数据采用NRTL模型得到的液体质量分数均方根偏差(RMSD)分别为0.013、0.018、和0.016,分别。此外,还计算了系统的KD值。
更新日期:2023-10-05
中文翻译:

使用三辛胺和稀释剂 2-乙基-1-己醇、4-甲基戊-2-酮和异戊醇从水溶液中反应萃取乙酰丙酸
从水溶液中分离羧酸是一项挑战,反应萃取已被视为一种有吸引力的替代方案。本研究研究了使用三辛胺 (TOA) 在各种稀释剂(例如 2-乙基-1-己醇、4-甲基戊-2-酮 (MIBK) 和异戊醇)中反应萃取乙酰丙酸 (LA)。为此,在T = 293.15 K 和大气压力下,通过实验获得了 LA + TOA + H 2 O + 稀释剂混合物的液液平衡 (LLE) 数据。根据获得的数据,根据分配系数( K D )评估各种 TOA/稀释剂混合物的能力。发现异戊醇是稀释区域 ( w LA aq < 0.1) 的有效稀释剂, K D值为 9.4。然而,增加 LA 浓度导致的萃取能力与其他测试的 TOA 稀释剂大致相同。此外,应用非随机双液(NRTL)过量吉布斯能量模型来关联联络线。上述2-乙基-1-己醇、MIBK、异戊醇不同LLE体系的实验数据采用NRTL模型得到的液体质量分数均方根偏差(RMSD)分别为0.013、0.018、和0.016,分别。此外,还计算了系统的KD值。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号