Tetrahedron Letters ( IF 1.5 ) Pub Date : 2023-10-05 , DOI: 10.1016/j.tetlet.2023.154784 Sowmy Adapa , Unati Sai Kodali , Amit Kumar Taneja , Vinu Bandaru , Bhuvan Tej Mandava , Bhagavatula Balakrishna , Bhagya Tej Mandava , Naresh Panigrahi , Mandava Venkata Basaveswara Rao , Manojit Pal
|
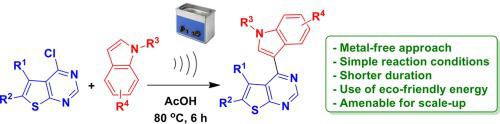
A sonochemical approach for the synthesis of 4-(1H-indol-3-yl)thieno[2,3-d]pyrimidine derivatives has been accomplished via the heteroarylation of 4-chloro thieno[2,3-d]pyrimidines with a range of indoles. The reaction was performed using AcOH as a promoter as well as solvent to give the corresponding products in good to acceptable yields. This metal free method proceeded via the C C bond forming reaction and offers advantages such as simple conditions, shorter duration and the use of eco-friendly energy. In addition to its drawbacks, the usefulness of the methodology is presented and exemplified by the medicinal value of the compounds synthesized. The compound 3i and 3j showed good inhibition of TNF-α in vitro and were identified as the initial hit molecules.
C bond forming reaction and offers advantages such as simple conditions, shorter duration and the use of eco-friendly energy. In addition to its drawbacks, the usefulness of the methodology is presented and exemplified by the medicinal value of the compounds synthesized. The compound 3i and 3j showed good inhibition of TNF-α in vitro and were identified as the initial hit molecules.
中文翻译:

AcOH介导的CC键形成反应超声辅助合成4-(1H-吲哚-3-基)噻吩并[2,3-d]嘧啶衍生物
通过4-氯噻吩并[2,3- d ]嘧啶与吲哚范围。该反应使用AcOH作为促进剂以及溶剂进行,以良好到可接受的收率得到相应的产物。这种无金属方法通过CC键形成反应进行,具有条件简单、持续时间短、使用环保能源等优点。除了其缺点之外,还通过合成的化合物的药用价值展示并举例说明了该方法的有用性。化合物3i和3j在体外表现出良好的TNF-α抑制作用,被确定为初始命中分子。































 京公网安备 11010802027423号
京公网安备 11010802027423号