Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Platinum–palladium-on-reduced graphene oxide as bifunctional electrocatalysts for highly active and stable hydrogen evolution and methanol oxidation reaction
Nanoscale ( IF 5.8 ) Pub Date : 2023-10-06 , DOI: 10.1039/d3nr04014c Yingliang Feng 1 , Lihua Zhu 1 , An Pei 1 , Sifan Zhang 1 , Kunming Liu 1 , Fengshun Wu 1 , Wenqi Li 1
Nanoscale ( IF 5.8 ) Pub Date : 2023-10-06 , DOI: 10.1039/d3nr04014c Yingliang Feng 1 , Lihua Zhu 1 , An Pei 1 , Sifan Zhang 1 , Kunming Liu 1 , Fengshun Wu 1 , Wenqi Li 1
Affiliation
|
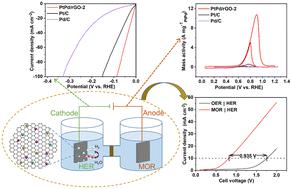
In the context of the gradual depletion of global fossil fuel resources, it is increasingly necessary to explore new alternative energy. Hydrogen energy has attracted great interest from researchers because of its green and pollution-free characteristics. Moreover, the methanol oxidation reaction (MOR) can combine the hydrogen evolution reaction (HER), replacing the anode reaction (oxygen evolution reaction-OER) in overall water splitting and efficiently producing hydrogen. In this study, platinum–palladium nanoparticles on reduced graphene oxide (PtPd/rGO) were successfully synthesized as HER and MOR bifunctional electrocatalysts under alkaline conditions by the stepwise loading of Pt and Pd bimetallic nanoparticles on rGO using a simple liquid-phase reduction method. PtPd/rGO-2 with 0.99 wt% Pt and 2.86 wt% Pd in the HER has the lowest overpotential (87.16 mV at 100 mA cm−2), with the smallest Tafel slope (18.9 mV dec−1). The exceptional mass activity of PtPd/rGO-2 in the MOR reaches 10.75 A mg−1PtPd, which is 18.22 and 53.75 times greater than that of commercial Pt/C (Pt/C) and commercial Pd/C (Pd/C), respectively. PtPd/rGO-2 is 0.935 V lower in the coupling reaction of HER and MOR (MOR ∥ HER) compared to the overall water splitting (OER ∥ HER) without methanol (10 mA cm−2). This is probably because appropriate Pt and Pd loading exposes many more catalytic sites, and the synergistic interaction between Pt, Pd, and Pt–Pd enhances the catalytic performance. This strategy can be used for the synthesis of novel bifunctional electrocatalysts.
中文翻译:

铂-钯还原氧化石墨烯作为双功能电催化剂,用于高活性和稳定的析氢和甲醇氧化反应
在全球化石燃料资源逐渐枯竭的背景下,探索新的替代能源日益必要。氢能以其绿色、无污染的特点引起了研究人员的极大兴趣。此外,甲醇氧化反应(MOR)可以结合析氢反应(HER),取代整体水分解中的阳极反应(析氧反应-OER),高效制氢。在本研究中,通过使用简单的液相还原方法在rGO上逐步负载Pt和Pd双金属纳米粒子,在碱性条件下成功合成了还原氧化石墨烯(PtPd/rGO)上的铂-钯纳米粒子作为HER和MOR双功能电催化剂。HER 中含有 0.99 wt% Pt 和 2.86 wt% Pd 的 PtPd/rGO-2 具有最低的过电势(100 mA cm -2 时为 87.16 mV),具有最小的塔菲尔斜率(18.9 mV dec -1)。PtPd/rGO-2在MOR中的卓越质量活性达到10.75 A mg −1 PtPd,分别是商业Pt/C(Pt/C)和商业Pd/C(Pd/C)的18.22和53.75倍, 分别。与没有甲醇(10 mA cm -2 )的总水分解(OER ∥ HER )相比,PtPd/rGO-2在HER和MOR的偶联反应(MOR ∥ HER)中低0.935 V。这可能是因为适当的 Pt 和 Pd 负载量暴露了更多的催化位点,并且 Pt、Pd 和 Pt-Pd 之间的协同相互作用增强了催化性能。该策略可用于合成新型双功能电催化剂。
更新日期:2023-10-06
中文翻译:

铂-钯还原氧化石墨烯作为双功能电催化剂,用于高活性和稳定的析氢和甲醇氧化反应
在全球化石燃料资源逐渐枯竭的背景下,探索新的替代能源日益必要。氢能以其绿色、无污染的特点引起了研究人员的极大兴趣。此外,甲醇氧化反应(MOR)可以结合析氢反应(HER),取代整体水分解中的阳极反应(析氧反应-OER),高效制氢。在本研究中,通过使用简单的液相还原方法在rGO上逐步负载Pt和Pd双金属纳米粒子,在碱性条件下成功合成了还原氧化石墨烯(PtPd/rGO)上的铂-钯纳米粒子作为HER和MOR双功能电催化剂。HER 中含有 0.99 wt% Pt 和 2.86 wt% Pd 的 PtPd/rGO-2 具有最低的过电势(100 mA cm -2 时为 87.16 mV),具有最小的塔菲尔斜率(18.9 mV dec -1)。PtPd/rGO-2在MOR中的卓越质量活性达到10.75 A mg −1 PtPd,分别是商业Pt/C(Pt/C)和商业Pd/C(Pd/C)的18.22和53.75倍, 分别。与没有甲醇(10 mA cm -2 )的总水分解(OER ∥ HER )相比,PtPd/rGO-2在HER和MOR的偶联反应(MOR ∥ HER)中低0.935 V。这可能是因为适当的 Pt 和 Pd 负载量暴露了更多的催化位点,并且 Pt、Pd 和 Pt-Pd 之间的协同相互作用增强了催化性能。该策略可用于合成新型双功能电催化剂。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号