Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2023-09-28 , DOI: 10.1016/j.molstruc.2023.136745 Alexey V. Dobrydnev , Maria V. Popova , Andrii V. Yatsymyrskyi , Svitlana V. Shishkina , Yaroslav O. Chuchvera , Yulian M. Volovenko
|
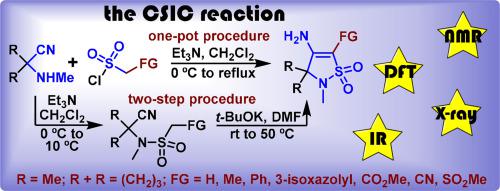
Herein we describe the reaction of 2-methyl-2-(methylamino)propanenitrile and 1-(methylamino)cyclobutane-1-carbonitrile with α-functionalized sulfonyl chlorides and the reactivity of resulting α-functionalized β-enamino γ-sultams. The nature of the substituent or the functional group in the α-position has a decisive impact on the reactivity of β-enamino γ-sultams. In particular, α-unsubstituted β-enamino γ-sultams and those possessing either electron-donating substituents or phenyl group are prepared through the two-step procedure and are prone to acid-mediated hydrolysis. Contrary to that, a strong electron-withdrawing group in the α-position enables the synthesis of the corresponding β-enamino γ-sultams in a one-pot manner and makes them tolerate the acid-mediated hydrolysis. The analysis of the results of a complex study involving physical methods (NMR and IR spectroscopy, X-ray diffraction study) and DFT calculations (performed at PBE0 QZVP level of theory) allowed us to rationalize and formulate the rules of structure‒activity relationship for the discussed sultams. It turned out, that the simplest way to predict the reactivity of α-functionalized β-enamino γ-sultams is the 1H NMR spectroscopy: if the NH2 group appears in the spectrum as a two-proton singlet the compound is highly likely can be further modified while the appearance of two separated one-proton singlets indicates a low reactivity of β-enamino γ-sultams.
中文翻译:

α-功能化β-烯胺γ-磺内酯结构-活性关系的合理化
在此,我们描述了2-甲基-2-(甲基氨基)丙腈和1-(甲基氨基)环丁烷-1-甲腈与α-官能化磺酰氯的反应以及所得α-官能化β-烯氨基γ-磺内酰胺的反应性。α位取代基或官能团的性质对β-烯胺γ-磺内酰胺的反应活性具有决定性影响。特别是,α-未取代的β-烯氨基γ-磺内酰胺和具有给电子取代基或苯基的那些是通过两步程序制备的,并且易于发生酸介导的水解。与此相反,α位上的强吸电子基团能够以一锅法合成相应的β-烯胺γ-磺内酰胺,并使它们能够耐受酸介导的水解。对涉及物理方法(核磁共振和红外光谱、X射线衍射研究)和DFT计算(在PBE0 QZVP理论水平上进行)的复杂研究结果的分析使我们能够合理化和制定结构-活性关系的规则所讨论的苏丹。事实证明,预测 α-功能化 β-enamino γ-sultams 反应性的最简单方法是1 H NMR 光谱:如果 NH 2基团以二质子单峰形式出现在光谱中,则该化合物很可能可以进一步修饰,而出现两个分离的一质子单峰则表明 β-烯胺 γ-磺内酰胺的反应性较低。








































 京公网安备 11010802027423号
京公网安备 11010802027423号