当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and neuroprotective activity of 3-aryl-3-azetidinyl acetic acid methyl ester derivatives
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2023-10-05 , DOI: 10.1002/ardp.202300378 Urtė Šachlevičiūtė 1 , Gabriel Gonzalez 2, 3 , Marie Kvasnicová 2 , Šárka Štěpánková 4 , Neringa Kleizienė 1 , Aurimas Bieliauskas 1 , Marek Zatloukal 5 , Miroslav Strnad 6 , Frank A Sløk 7 , Miroslav Kvasnica 6 , Algirdas Šačkus 1 , Asta Žukauskaitė 5
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2023-10-05 , DOI: 10.1002/ardp.202300378 Urtė Šachlevičiūtė 1 , Gabriel Gonzalez 2, 3 , Marie Kvasnicová 2 , Šárka Štěpánková 4 , Neringa Kleizienė 1 , Aurimas Bieliauskas 1 , Marek Zatloukal 5 , Miroslav Strnad 6 , Frank A Sløk 7 , Miroslav Kvasnica 6 , Algirdas Šačkus 1 , Asta Žukauskaitė 5
Affiliation
|
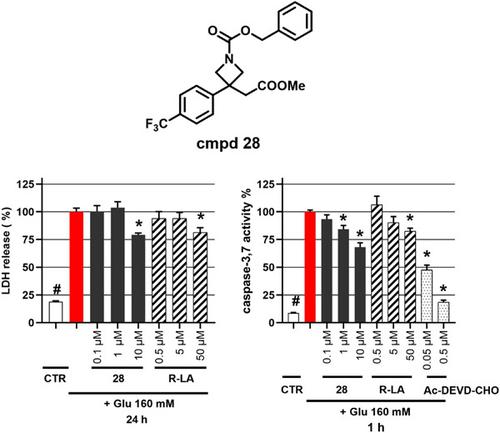
A library of 3-aryl-3-azetidinyl acetic acid methyl ester derivatives was prepared from N-Boc-3-azetidinone employing the Horner-Wadsworth-Emmons reaction, rhodium(I)-catalyzed conjugate addition of arylboronic acids, and subsequent elaborations to obtain N-unprotected hydrochlorides, N-alkylated and N-acylated azetidine derivatives. The compounds were evaluated for acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activity, revealing several derivatives to possess AChE inhibition comparable to that of the AChE inhibitor rivastigmine. The binding mode of the AChE inhibitor donepezil and selected active compounds 26 and 27 within the active site of AChE was studied using molecular docking. Furthermore, the neuroprotective activity of the prepared compounds was evaluated in models associated with Parkinson's disease (salsolinol-induced) and aspects of Alzheimer's disease (glutamate-induced oxidative damage). Compound 28 showed the highest neuroprotective effect in both salsolinol- and glutamate-induced neurodegeneration models, and its protective effect in the glutamate model was revealed to be driven by a reduction in oxidative stress and caspase-3/7 activity.
中文翻译:

3-芳基-3-氮杂环丁烷基乙酸甲酯衍生物的合成及其神经保护活性
采用 Horner-Wadsworth-Emmons 反应、铑 (I) 催化的芳基硼酸共轭加成,以及随后的精制,由N -Boc-3-氮杂环丁酮制备了 3-芳基-3-氮杂环丁基乙酸甲酯衍生物库。获得N-未保护的盐酸盐、 N-烷基化和N-酰化氮杂环丁烷衍生物。对这些化合物的乙酰胆碱酯酶 (AChE) 和丁酰胆碱酯酶 (BChE) 抑制活性进行了评估,结果表明几种衍生物具有与 AChE 抑制剂卡巴拉汀 (rivastigmine) 相当的 AChE 抑制作用。使用分子对接研究了 AChE 抑制剂多奈哌齐和所选活性化合物26和27在 AChE 活性位点内的结合模式。此外,在与帕金森病(salsolinol诱导的)和阿尔茨海默病(谷氨酸诱导的氧化损伤)相关的模型中评估了所制备的化合物的神经保护活性。化合物28在猪油醇和谷氨酸诱导的神经变性模型中均表现出最高的神经保护作用,并且其在谷氨酸模型中的保护作用被揭示是由氧化应激和 caspase-3/7 活性的降低驱动的。
更新日期:2023-10-05
中文翻译:

3-芳基-3-氮杂环丁烷基乙酸甲酯衍生物的合成及其神经保护活性
采用 Horner-Wadsworth-Emmons 反应、铑 (I) 催化的芳基硼酸共轭加成,以及随后的精制,由N -Boc-3-氮杂环丁酮制备了 3-芳基-3-氮杂环丁基乙酸甲酯衍生物库。获得N-未保护的盐酸盐、 N-烷基化和N-酰化氮杂环丁烷衍生物。对这些化合物的乙酰胆碱酯酶 (AChE) 和丁酰胆碱酯酶 (BChE) 抑制活性进行了评估,结果表明几种衍生物具有与 AChE 抑制剂卡巴拉汀 (rivastigmine) 相当的 AChE 抑制作用。使用分子对接研究了 AChE 抑制剂多奈哌齐和所选活性化合物26和27在 AChE 活性位点内的结合模式。此外,在与帕金森病(salsolinol诱导的)和阿尔茨海默病(谷氨酸诱导的氧化损伤)相关的模型中评估了所制备的化合物的神经保护活性。化合物28在猪油醇和谷氨酸诱导的神经变性模型中均表现出最高的神经保护作用,并且其在谷氨酸模型中的保护作用被揭示是由氧化应激和 caspase-3/7 活性的降低驱动的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号