当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of 2-Aminothiazole-4-carboxylic Acids as Broad-Spectrum Metallo-β-lactamase Inhibitors by Mimicking Carbapenem Hydrolysate Binding
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-10-04 , DOI: 10.1021/acs.jmedchem.3c01189 Yu-Hang Yan 1 , Ting-Ting Zhang 2 , Rong Li 1, 3 , Si-Yao Wang 1 , Liu-Liu Wei 1 , Xin-Yue Wang 1 , Kai-Rong Zhu 1 , Shan-Rui Li 1 , Guo-Qing Liang 1 , Zeng-Bao Yang 1 , Ling-Ling Yang 3 , Shangshang Qin 2 , Guo-Bo Li 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-10-04 , DOI: 10.1021/acs.jmedchem.3c01189 Yu-Hang Yan 1 , Ting-Ting Zhang 2 , Rong Li 1, 3 , Si-Yao Wang 1 , Liu-Liu Wei 1 , Xin-Yue Wang 1 , Kai-Rong Zhu 1 , Shan-Rui Li 1 , Guo-Qing Liang 1 , Zeng-Bao Yang 1 , Ling-Ling Yang 3 , Shangshang Qin 2 , Guo-Bo Li 1
Affiliation
|
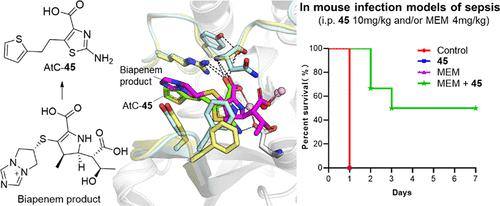
Metallo-β-lactamases (MBLs) are zinc-dependent enzymes capable of hydrolyzing all bicyclic β-lactam antibiotics, posing a great threat to public health. However, there are currently no clinically approved MBL inhibitors. Despite variations in their active sites, MBLs share a common catalytic mechanism with carbapenems, forming similar reaction species and hydrolysates. We here report the development of 2-aminothiazole-4-carboxylic acids (AtCs) as broad-spectrum MBL inhibitors by mimicking the anchor pharmacophore features of carbapenem hydrolysate binding. Several AtCs manifested potent activity against B1, B2, and B3 MBLs. Crystallographic analyses revealed a common binding mode of AtCs with B1, B2, and B3 MBLs, resembling binding observed in the MBL-carbapenem product complexes. AtCs restored Meropenem activity against MBL-producing isolates. In the murine sepsis model, AtCs exhibited favorable synergistic efficacy with Meropenem, along with acceptable pharmacokinetics and safety profiles. This work offers promising lead compounds and a structural basis for the development of potential drug candidates to combat MBL-mediated antimicrobial resistance.
中文翻译:

通过模拟碳青霉烯水解产物结合发现 2-氨基噻唑-4-羧酸作为广谱金属-β-内酰胺酶抑制剂
金属-β-内酰胺酶(MBL)是锌依赖性酶,能够水解所有双环β-内酰胺抗生素,对公众健康构成巨大威胁。然而,目前尚无临床批准的MBL抑制剂。尽管活性位点不同,MBL 与碳青霉烯类具有共同的催化机制,形成相似的反应物种和水解产物。我们在这里报告了通过模仿碳青霉烯水解产物结合的锚定药效团特征,开发了 2-氨基噻唑-4-羧酸 (AtC) 作为广谱 MBL 抑制剂。一些 AtC 对 B1、B2 和 B3 MBL 表现出有效的活性。晶体学分析揭示了 AtC 与 B1、B2 和 B3 MBL 的常见结合模式,类似于在 MBL-碳青霉烯产物复合物中观察到的结合。AtCs 恢复了美罗培南针对产生 MBL 的分离株的活性。在小鼠脓毒症模型中,AtCs 与美罗培南表现出良好的协同功效,以及可接受的药代动力学和安全性。这项工作为开发对抗 MBL 介导的抗菌药物耐药性的潜在候选药物提供了有前景的先导化合物和结构基础。
更新日期:2023-10-04
中文翻译:

通过模拟碳青霉烯水解产物结合发现 2-氨基噻唑-4-羧酸作为广谱金属-β-内酰胺酶抑制剂
金属-β-内酰胺酶(MBL)是锌依赖性酶,能够水解所有双环β-内酰胺抗生素,对公众健康构成巨大威胁。然而,目前尚无临床批准的MBL抑制剂。尽管活性位点不同,MBL 与碳青霉烯类具有共同的催化机制,形成相似的反应物种和水解产物。我们在这里报告了通过模仿碳青霉烯水解产物结合的锚定药效团特征,开发了 2-氨基噻唑-4-羧酸 (AtC) 作为广谱 MBL 抑制剂。一些 AtC 对 B1、B2 和 B3 MBL 表现出有效的活性。晶体学分析揭示了 AtC 与 B1、B2 和 B3 MBL 的常见结合模式,类似于在 MBL-碳青霉烯产物复合物中观察到的结合。AtCs 恢复了美罗培南针对产生 MBL 的分离株的活性。在小鼠脓毒症模型中,AtCs 与美罗培南表现出良好的协同功效,以及可接受的药代动力学和安全性。这项工作为开发对抗 MBL 介导的抗菌药物耐药性的潜在候选药物提供了有前景的先导化合物和结构基础。






























 京公网安备 11010802027423号
京公网安备 11010802027423号