当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Efficient H2O2 Production via Two-Electron Electrochemical Oxygen Reduction over Fe-Doped CeO2
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-10-04 , DOI: 10.1021/acssuschemeng.3c04194
Xueli Mei 1 , Xueyang Zhao 2 , Yaoyao Chen 1 , Bangwei Deng 2 , Qin Geng 2 , Yali Cao 1 , Yizhao Li 1, 2 , Fan Dong 2, 3
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-10-04 , DOI: 10.1021/acssuschemeng.3c04194
Xueli Mei 1 , Xueyang Zhao 2 , Yaoyao Chen 1 , Bangwei Deng 2 , Qin Geng 2 , Yali Cao 1 , Yizhao Li 1, 2 , Fan Dong 2, 3
Affiliation
|
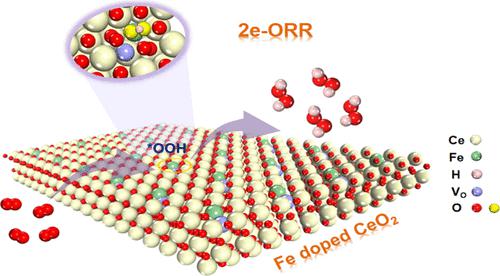
Electrochemical two-electron oxygen reduction reaction (2e-ORR) is regarded as a green replacement to traditional anthraquinone processes for the continuous on-site production of H2O2. Low-cost, highly selective, and active catalysts are needed for the process. In this work, we report that Fe-doped CeO2 (Fe-CeO2) can be used as an effective catalyst for the synthesis of H2O2, which exhibits high 2e-ORR performance relative to pristine CeO2. This is because the doping of the Fe leads to lattice distortion of CeO2 and further formation of many oxygen vacancies and Ce3+, which contributes to improving the activity of Fe–CeO2 electrocatalysts for 2e-ORR. It was found that the amount of Fe doping and temperature of heat treatment have an effect on the oxygen vacancies of Fe–CeO2, which in turn affects the performance of the 2e-ORR. The prepared catalyst (Fe–CeO2-3) showed optimal 2e-ORR performance when the molar ratio of ferric nitrate to cerium nitrate was 0.3 and the calcination temperature was 600 °C. The catalyst showed a selectivity of up to 97.7% for H2O2 at 0.38 V (vs RHE) in 0.1 M KOH solution, which is much superior to the pristine CeO2 (53%). Additionally, compared with original CeO2, the H2O2 yield of Fe–CeO2-3 electrocatalyst was greatly improved in the electrolysis process in an H-cell device, reaching 1.80 mol gcat–1 h–1 at 0.1 V (vs RHE) with a high Faraday efficiency of 94%. Density functional theory calculations demonstrate that Fe doped on CeO2 lowers free energy barrier for the formation of *OOH intermediate, thus facilitating H2O2 formation. Our work proposes a facile approach to develop effective nonnoble metal catalysts for electrochemical production of H2O2 by 2e-ORR.
中文翻译:

通过 Fe 掺杂 CeO2 上的双电子电化学氧还原高效生产 H2O2
电化学双电子氧还原反应(2e-ORR)被认为是传统蒽醌法连续现场生产H 2 O 2的绿色替代方法。该过程需要低成本、高选择性和活性的催化剂。在这项工作中,我们报道了Fe掺杂的CeO 2 (Fe-CeO 2 )可以用作合成H 2 O 2的有效催化剂,相对于原始CeO 2表现出较高的2e-ORR性能。这是因为Fe的掺杂导致CeO 2的晶格畸变,并进一步形成许多氧空位和Ce 3+,这有助于提高Fe-CeO 2电催化剂的2e-ORR活性。研究发现Fe掺杂量和热处理温度对Fe-CeO 2的氧空位有影响,进而影响2e-ORR的性能。当硝酸铁与硝酸铈的摩尔比为0.3、煅烧温度为600℃时,所制备的催化剂(Fe–CeO 2 -3)表现出最佳的2e-ORR性能。该催化剂在0.1 M KOH溶液中、0.38 V(vs RHE)下对H 2 O 2的选择性高达97.7% ,远远优于原始CeO 2 (53%)。此外,与原始CeO 2相比,Fe-CeO 2 -3 电催化剂在H电池装置电解过程中的H 2 O 2产率大大提高,在0.1 V下达到1.80 mol g cat –1 h –1 ( vs RHE),法拉第效率高达 94%。密度泛函理论计算表明,CeO 2上掺杂的Fe降低了*OOH中间体形成的自由能垒,从而促进H 2 O 2的形成。我们的工作提出了一种简便的方法来开发有效的非贵金属催化剂,用于通过 2e-ORR电化学生产 H 2 O 2 。
更新日期:2023-10-04
中文翻译:

通过 Fe 掺杂 CeO2 上的双电子电化学氧还原高效生产 H2O2
电化学双电子氧还原反应(2e-ORR)被认为是传统蒽醌法连续现场生产H 2 O 2的绿色替代方法。该过程需要低成本、高选择性和活性的催化剂。在这项工作中,我们报道了Fe掺杂的CeO 2 (Fe-CeO 2 )可以用作合成H 2 O 2的有效催化剂,相对于原始CeO 2表现出较高的2e-ORR性能。这是因为Fe的掺杂导致CeO 2的晶格畸变,并进一步形成许多氧空位和Ce 3+,这有助于提高Fe-CeO 2电催化剂的2e-ORR活性。研究发现Fe掺杂量和热处理温度对Fe-CeO 2的氧空位有影响,进而影响2e-ORR的性能。当硝酸铁与硝酸铈的摩尔比为0.3、煅烧温度为600℃时,所制备的催化剂(Fe–CeO 2 -3)表现出最佳的2e-ORR性能。该催化剂在0.1 M KOH溶液中、0.38 V(vs RHE)下对H 2 O 2的选择性高达97.7% ,远远优于原始CeO 2 (53%)。此外,与原始CeO 2相比,Fe-CeO 2 -3 电催化剂在H电池装置电解过程中的H 2 O 2产率大大提高,在0.1 V下达到1.80 mol g cat –1 h –1 ( vs RHE),法拉第效率高达 94%。密度泛函理论计算表明,CeO 2上掺杂的Fe降低了*OOH中间体形成的自由能垒,从而促进H 2 O 2的形成。我们的工作提出了一种简便的方法来开发有效的非贵金属催化剂,用于通过 2e-ORR电化学生产 H 2 O 2 。

































 京公网安备 11010802027423号
京公网安备 11010802027423号