Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ammonium Persulfate-Loaded Carboxylic Gelatin–Methacrylate Nanoparticles Promote Cardiac Repair by Activating Epicardial Epithelial–Mesenchymal Transition via Autophagy and the mTOR Pathway
ACS Nano ( IF 15.8 ) Pub Date : 2023-10-02 , DOI: 10.1021/acsnano.3c06229 Chen Song 1 , Fanxuan Kong 2 , Huijia Nong 3 , Liu Cai 3 , Ye Tian 3 , Honghao Hou 2 , Leyu Wang 3 , Xiaozhong Qiu 1, 2
ACS Nano ( IF 15.8 ) Pub Date : 2023-10-02 , DOI: 10.1021/acsnano.3c06229 Chen Song 1 , Fanxuan Kong 2 , Huijia Nong 3 , Liu Cai 3 , Ye Tian 3 , Honghao Hou 2 , Leyu Wang 3 , Xiaozhong Qiu 1, 2
Affiliation
|
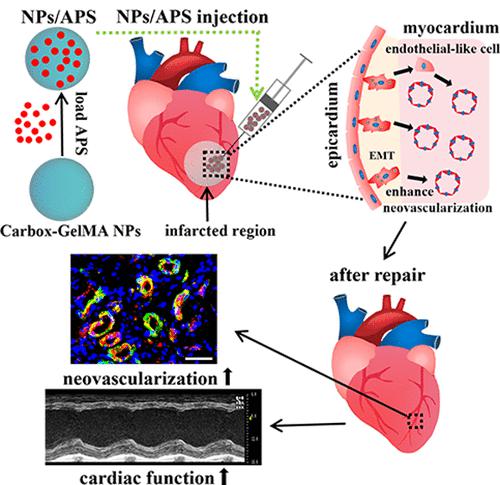
Restoring damaged myocardial tissue with therapeutic exogenous cells still has some limitations, such as immunological rejection, immature cardiac properties, risk of tumorigenicity, and a low cell survival rate in the ischemic myocardium microenvironment. Activating the endogenous stem cells with functional biomaterials might overcome these limitations. Research has highlighted the multiple differentiation potential of epicardial cells via epithelial–mesenchymal transition (EMT) in both heart development and cardiac regeneration. In our previous research, a carboxylic gelatin–methacrylate (carbox-GelMA) nanoparticle (NP) was fabricated to carry ammonium persulfate (APS), and APS-loaded carbox-GelMA NPs (NPs/APS) could drive the EMT of MCF-7 cells in vitro and promote cancer cell migration and invasion in vivo. The present study explored the roles of functional NPs/APS in the EMT of Wilms’ tumor 1-positive (WT1+) epicardial cells and in the repair of myocardial infarction (MI). The WT1+ epicardial cells transformed into endothelial-like cells after being treated with NPs/APS in vitro, and the cardiac functions were improved significantly after injecting NPs/APS into the infarcted hearts in vivo. Furthermore, simultaneous activation of both autophagy and the mTOR pathway was confirmed during the NPs/APS-induced EMT process in WT1+ epicardial cells. Together, this study highlights the function of NPs/APS in the repair of MI.
中文翻译:

负载过硫酸铵的羧基明胶-甲基丙烯酸酯纳米颗粒通过自噬和 mTOR 途径激活心外膜上皮-间质转化,促进心脏修复
用治疗性外源细胞修复受损心肌组织仍存在一些局限性,如免疫排斥、心脏特性不成熟、致瘤风险以及缺血心肌微环境中细胞存活率低等。用功能性生物材料激活内源干细胞可能会克服这些限制。研究强调了心外膜细胞通过上皮间质转化(EMT)在心脏发育和心脏再生中的多重分化潜力。在我们之前的研究中,制备了一种羧基明胶-甲基丙烯酸酯(carbox-GelMA)纳米颗粒(NP)来承载过硫酸铵(APS),负载APS的carbox-GelMA NP(NPs/APS)可以驱动MCF-7的EMT体外抑制细胞生长,促进体内癌细胞迁移和侵袭。本研究探讨了功能性 NPs/APS 在 Wilms 肿瘤 1 阳性 (WT1 + ) 心外膜细胞的 EMT 和心肌梗死 (MI) 修复中的作用。WT1 +心外膜细胞经NPs/APS体外处理后转化为内皮样细胞,体内注射NPs/APS至梗死心脏后心脏功能明显改善。此外,在 WT1 +心外膜细胞中 NPs/APS 诱导的 EMT 过程中,证实了自噬和 mTOR 通路的同时激活。总之,这项研究强调了 NPs/APS 在 MI 修复中的功能。
更新日期:2023-10-02
中文翻译:

负载过硫酸铵的羧基明胶-甲基丙烯酸酯纳米颗粒通过自噬和 mTOR 途径激活心外膜上皮-间质转化,促进心脏修复
用治疗性外源细胞修复受损心肌组织仍存在一些局限性,如免疫排斥、心脏特性不成熟、致瘤风险以及缺血心肌微环境中细胞存活率低等。用功能性生物材料激活内源干细胞可能会克服这些限制。研究强调了心外膜细胞通过上皮间质转化(EMT)在心脏发育和心脏再生中的多重分化潜力。在我们之前的研究中,制备了一种羧基明胶-甲基丙烯酸酯(carbox-GelMA)纳米颗粒(NP)来承载过硫酸铵(APS),负载APS的carbox-GelMA NP(NPs/APS)可以驱动MCF-7的EMT体外抑制细胞生长,促进体内癌细胞迁移和侵袭。本研究探讨了功能性 NPs/APS 在 Wilms 肿瘤 1 阳性 (WT1 + ) 心外膜细胞的 EMT 和心肌梗死 (MI) 修复中的作用。WT1 +心外膜细胞经NPs/APS体外处理后转化为内皮样细胞,体内注射NPs/APS至梗死心脏后心脏功能明显改善。此外,在 WT1 +心外膜细胞中 NPs/APS 诱导的 EMT 过程中,证实了自噬和 mTOR 通路的同时激活。总之,这项研究强调了 NPs/APS 在 MI 修复中的功能。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号