当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Current Collector Design to Accelerate LiNO3 Decomposition for High-Performing Lithium Metal Batteries
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2023-10-03 , DOI: 10.1002/aenm.202302620
Qicheng Zhang 1, 2 , Lei Xu 3 , Xinyang Yue 1 , Jijiang Liu 1 , Xin Wang 4 , Xiaoya He 1 , Zidan Shi 1 , Shuzhang Niu 4 , Wei Gao 2 , Chun Cheng 3 , Zheng Liang 1
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2023-10-03 , DOI: 10.1002/aenm.202302620
Qicheng Zhang 1, 2 , Lei Xu 3 , Xinyang Yue 1 , Jijiang Liu 1 , Xin Wang 4 , Xiaoya He 1 , Zidan Shi 1 , Shuzhang Niu 4 , Wei Gao 2 , Chun Cheng 3 , Zheng Liang 1
Affiliation
|
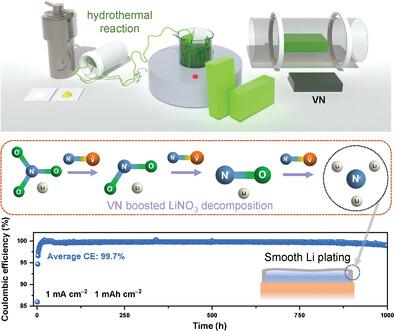
Lithium nitrate is an attractive lithium additive in the construction of high-performance lithium metal anodes with a Li3N-rich solid electrolyte interphase (SEI) layer. However, the eight-electron transfer process induces high energy barriers between LiNO3 and Li3N. Herein, the inner Helmholtz plane is tuned on a Li deposition host to attain sluggish/rapid LiNO3 decomposition kinetics, resulting in different intermediate content distributions of Li species in the SEI. Notably, lithium oxynitride (LiNO) is identified as the decomposition intermediate, and experimental and simulation results confirm its role in obstructing LiNO3 decomposition. Moreover, the results reveal that the dipole–dipole interaction between LiNO and the polar V≡N bond can change the ionic/covalent character of the N═O bonds, considerably facilitating the energy transfer process of the N═O cleavage, and promoting a LiNO3 reduction to achieve a Li3N-rich SEI. Consequently, when the electrolyte contains 0.37 m LiNO3, dendrite, and dead Li formation are suppressed effectively with the VN system, and an average Coulombic efficiency of 99.7% over 1000 cycles (1 mA cm−2, 1 mAh cm−2) can be attained. These results can promote the nitride oxidation break process and pave the way for fabricating high-performance Li3N-rich lithium metal batteries.
中文翻译:

加速高性能锂金属电池 LiNO3 分解的催化集电器设计
硝酸锂是一种极具吸引力的锂添加剂,可用于构建具有富含 Li 3 N 的固体电解质中间相 (SEI) 层的高性能锂金属阳极。然而,八电子转移过程在LiNO 3和Li 3 N之间引起高能垒。本文中,在Li沉积主体上调整内亥姆霍兹平面以获得缓慢/快速的LiNO 3分解动力学,从而产生不同的中间含量分布SEI中的Li种。值得注意的是,氮氧化锂(LiNO)被确定为分解中间体,实验和模拟结果证实了其在阻碍LiNO 3分解中的作用。此外,结果表明,LiNO与极性V=N键之间的偶极-偶极相互作用可以改变N=O键的离子/共价特性,极大地促进N=O裂解的能量转移过程,并促进LiNO 3还原以实现富含Li 3 N的SEI。因此,当电解液中含有0.37 m LiNO 3时,VN系统可以有效抑制枝晶和死锂的形成,并且1000次循环(1 mA cm -2 、 1 mAh cm -2)的平均库仑效率为99.7%。得以实现。这些结果可以促进氮化物氧化破坏过程,为制造高性能Li 3 N富锂金属电池铺平道路。
更新日期:2023-10-03
中文翻译:

加速高性能锂金属电池 LiNO3 分解的催化集电器设计
硝酸锂是一种极具吸引力的锂添加剂,可用于构建具有富含 Li 3 N 的固体电解质中间相 (SEI) 层的高性能锂金属阳极。然而,八电子转移过程在LiNO 3和Li 3 N之间引起高能垒。本文中,在Li沉积主体上调整内亥姆霍兹平面以获得缓慢/快速的LiNO 3分解动力学,从而产生不同的中间含量分布SEI中的Li种。值得注意的是,氮氧化锂(LiNO)被确定为分解中间体,实验和模拟结果证实了其在阻碍LiNO 3分解中的作用。此外,结果表明,LiNO与极性V=N键之间的偶极-偶极相互作用可以改变N=O键的离子/共价特性,极大地促进N=O裂解的能量转移过程,并促进LiNO 3还原以实现富含Li 3 N的SEI。因此,当电解液中含有0.37 m LiNO 3时,VN系统可以有效抑制枝晶和死锂的形成,并且1000次循环(1 mA cm -2 、 1 mAh cm -2)的平均库仑效率为99.7%。得以实现。这些结果可以促进氮化物氧化破坏过程,为制造高性能Li 3 N富锂金属电池铺平道路。

































 京公网安备 11010802027423号
京公网安备 11010802027423号