当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein Phosphorylation Induced by Pyruvate Kinase M2 Inhibited Myofibrillar Protein Degradation in Post-Mortem Muscle
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2023-09-30 , DOI: 10.1021/acs.jafc.3c03930 Chi Ren 1, 2 , Xubo Song 1 , Yu Dong 1 , Chengli Hou 1 , Li Chen 1 , Zhenyu Wang 1 , Xin Li 1 , Martine Schroyen 2 , Dequan Zhang 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2023-09-30 , DOI: 10.1021/acs.jafc.3c03930 Chi Ren 1, 2 , Xubo Song 1 , Yu Dong 1 , Chengli Hou 1 , Li Chen 1 , Zhenyu Wang 1 , Xin Li 1 , Martine Schroyen 2 , Dequan Zhang 1
Affiliation
|
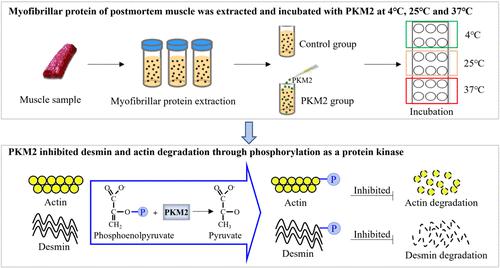
Myofibrillar protein degradation is primarily related to meat tenderness through protein phosphorylation regulation. Pyruvate kinase M2 (PKM2), a glycolytic rate-limiting enzyme, is also regarded as a protein kinase to catalyze phosphorylation. The objective of this study was to investigate the relationship between myofibrillar protein degradation and phosphorylation induced by PKM2. Myofibrillar proteins were incubated with PKM2 at 4, 25, and 37 °C. The global phosphorylation level of myofibrillar proteins in the PKM2 group was significantly increased, but it was sensitive to temperature (P < 0.05). Compared with 4 and 25 °C, PKM2 significantly increased the myofibrillar protein phosphorylation level from 0.5 to 6 h at 37 °C (P < 0.05). In addition, the degradation of desmin and actin was inhibited after they were phosphorylated by PKM2 when incubated at 37 °C. These results demonstrate that phosphorylation of myofibrillar proteins catalyzed by PKM2 inhibited protein degradation and provided a possible pathway for meat tenderization through glycolytic enzyme regulation.
中文翻译:

丙酮酸激酶 M2 诱导的蛋白质磷酸化抑制死后肌肉中的肌原纤维蛋白降解
肌原纤维蛋白降解主要通过蛋白磷酸化调节与肉嫩度相关。丙酮酸激酶M2(PKM2)是一种糖酵解限速酶,也被认为是催化磷酸化的蛋白激酶。本研究的目的是探讨 PKM2 诱导的肌原纤维蛋白降解与磷酸化之间的关系。肌原纤维蛋白与 PKM2 在 4、25 和 37 °C 下孵育。PKM2 组肌原纤维蛋白整体磷酸化水平显着升高,但对温度敏感(P < 0.05)。与4和25℃相比,PKM2在37℃下0.5至6小时显着增加肌原纤维蛋白磷酸化水平(P <0.05)。此外,在37℃孵育时,结蛋白和肌动蛋白被PKM2磷酸化后,其降解受到抑制。这些结果表明,PKM2 催化的肌原纤维蛋白磷酸化抑制了蛋白质降解,并通过糖酵解酶调节为肉嫩化提供了可能的途径。
更新日期:2023-09-30
中文翻译:

丙酮酸激酶 M2 诱导的蛋白质磷酸化抑制死后肌肉中的肌原纤维蛋白降解
肌原纤维蛋白降解主要通过蛋白磷酸化调节与肉嫩度相关。丙酮酸激酶M2(PKM2)是一种糖酵解限速酶,也被认为是催化磷酸化的蛋白激酶。本研究的目的是探讨 PKM2 诱导的肌原纤维蛋白降解与磷酸化之间的关系。肌原纤维蛋白与 PKM2 在 4、25 和 37 °C 下孵育。PKM2 组肌原纤维蛋白整体磷酸化水平显着升高,但对温度敏感(P < 0.05)。与4和25℃相比,PKM2在37℃下0.5至6小时显着增加肌原纤维蛋白磷酸化水平(P <0.05)。此外,在37℃孵育时,结蛋白和肌动蛋白被PKM2磷酸化后,其降解受到抑制。这些结果表明,PKM2 催化的肌原纤维蛋白磷酸化抑制了蛋白质降解,并通过糖酵解酶调节为肉嫩化提供了可能的途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号