Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing Electroreduction CO2 to Hydrocarbons via Tandem Electrocatalysis by Incorporation Cu NPs in Boron Imidazolate Frameworks
Small ( IF 13.0 ) Pub Date : 2023-09-29 , DOI: 10.1002/smll.202305199 Ping Shao 1, 2 , Yu-Mei Wan 2 , Luocai Yi 2 , Shumei Chen 1, 2 , Hai-Xia Zhang 2 , Jian Zhang 2
Small ( IF 13.0 ) Pub Date : 2023-09-29 , DOI: 10.1002/smll.202305199 Ping Shao 1, 2 , Yu-Mei Wan 2 , Luocai Yi 2 , Shumei Chen 1, 2 , Hai-Xia Zhang 2 , Jian Zhang 2
Affiliation
|
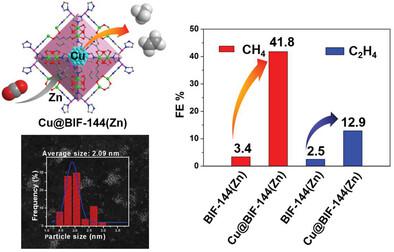
Due to the higher value of deeply-reduced products, electrocatalytic CO2 reduction reaction (CO2RR) to multi-electron-transfer products has received more attention. One attractive strategy is to decouple individual steps within the complicated pathway via multi-component catalysts design in the concept of tandem catalysts. Here, a composite of Cu@BIF-144(Zn) (BIF = boron imidazolate framework) is synthesized by using an anion framework BIF-144(Zn) as host to impregnate Cu2+ ions that are further reduced to Cu nanoparticles (NPs) via in situ electrochemical transformation. Due to the microenvironment modulation by functional BH(im)3− on the pore surfaces, the Cu@BIF-144(Zn) catalyst exhibits a perfect synergetic effect between the BIF-144(Zn) host and the Cu NP guest during CO2RR. Electrochemistry results show that Cu@BIF-144(Zn) catalysts can effectively enhance the selectivity and activity for the CO2 reduction to multi-electron-transfer products, with the maximum FECH4 value of 41.8% at −1.6 V and FEC2H4 value of 12.9% at −1.5 V versus RHE. The Cu@BIF-144(Zn) tandem catalyst with CO-rich microenvironment generated by the Zn catalytic center in the BIF-144(Zn) skeleton enhanced deep reduction on the incorporated Cu NPs for the CO2RR to multi-electron-transfer products.
中文翻译:

通过在硼咪唑框架中掺入 Cu NP 来增强通过串联电催化将 CO2 电还原为碳氢化合物
由于深度还原产物的价值较高,电催化CO 2还原反应(CO 2 RR)生成多电子转移产物受到越来越多的关注。一种有吸引力的策略是通过串联催化剂概念中的多组分催化剂设计来解耦复杂途径中的各个步骤。在这里,通过使用阴离子骨架 BIF-144(Zn) 作为主体来浸渍 Cu 2+离子,进一步还原为 Cu 纳米颗粒(NPs),合成了 Cu@BIF-144(Zn)(BIF = 硼咪唑骨架)的复合材料。 )通过原位电化学转化。由于孔表面功能性BH(im) 3 −的微环境调节,Cu@BIF-144(Zn)催化剂在CO 2过程中表现出BIF-144(Zn)主体和Cu NP客体之间完美的协同效应RR。电化学结果表明Cu@BIF-144(Zn)催化剂能有效提高CO 2还原为多电子转移产物的选择性和活性,在-1.6 V和FE C2H4值下最大FE CH4值为41.8%与 RHE 相比,-1.5 V 时为 12.9%。 Cu@BIF-144(Zn)串联催化剂具有由BIF-144(Zn)骨架中的Zn催化中心产生的富含CO的微环境,增强了掺入的Cu NPs的深度还原,从而实现CO 2 RR多电子转移产品。
更新日期:2023-09-29
中文翻译:

通过在硼咪唑框架中掺入 Cu NP 来增强通过串联电催化将 CO2 电还原为碳氢化合物
由于深度还原产物的价值较高,电催化CO 2还原反应(CO 2 RR)生成多电子转移产物受到越来越多的关注。一种有吸引力的策略是通过串联催化剂概念中的多组分催化剂设计来解耦复杂途径中的各个步骤。在这里,通过使用阴离子骨架 BIF-144(Zn) 作为主体来浸渍 Cu 2+离子,进一步还原为 Cu 纳米颗粒(NPs),合成了 Cu@BIF-144(Zn)(BIF = 硼咪唑骨架)的复合材料。 )通过原位电化学转化。由于孔表面功能性BH(im) 3 −的微环境调节,Cu@BIF-144(Zn)催化剂在CO 2过程中表现出BIF-144(Zn)主体和Cu NP客体之间完美的协同效应RR。电化学结果表明Cu@BIF-144(Zn)催化剂能有效提高CO 2还原为多电子转移产物的选择性和活性,在-1.6 V和FE C2H4值下最大FE CH4值为41.8%与 RHE 相比,-1.5 V 时为 12.9%。 Cu@BIF-144(Zn)串联催化剂具有由BIF-144(Zn)骨架中的Zn催化中心产生的富含CO的微环境,增强了掺入的Cu NPs的深度还原,从而实现CO 2 RR多电子转移产品。

































 京公网安备 11010802027423号
京公网安备 11010802027423号