当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Methylated Nucleotide-Based Proteolysis-Targeting Chimera Enables Targeted Degradation of Methyl-CpG-Binding Protein 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-09-29 , DOI: 10.1021/jacs.3c06023
Zhen Wang 1 , Jing Liu 1 , Xing Qiu 2 , Dingpeng Zhang 3 , Hiroyuki Inuzuka 1 , Li Chen 1 , He Chen 2 , Ling Xie 4, 5 , H Ümit Kaniskan 2 , Xian Chen 4, 5 , Jian Jin 2 , Wenyi Wei 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-09-29 , DOI: 10.1021/jacs.3c06023
Zhen Wang 1 , Jing Liu 1 , Xing Qiu 2 , Dingpeng Zhang 3 , Hiroyuki Inuzuka 1 , Li Chen 1 , He Chen 2 , Ling Xie 4, 5 , H Ümit Kaniskan 2 , Xian Chen 4, 5 , Jian Jin 2 , Wenyi Wei 1
Affiliation
|
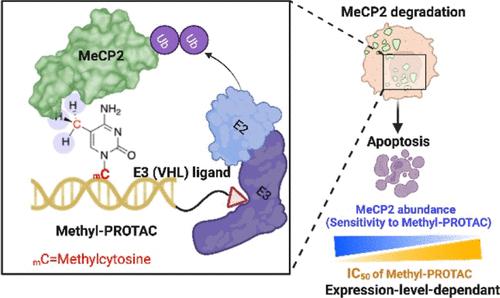
Methyl-CpG-binding protein 2 (MeCP2), a reader of DNA methylation, has been extensively investigated for its function in neurological and neurodevelopmental disorders. Emerging evidence indicates that MeCP2 exerts an oncogenic function in cancer; however, the endeavor to develop a MeCP2-targeted therapy remains a challenge. This work attempts to address it by introducing a methylated nucleotide-based targeting chimera termed methyl-proteolysis-targeting chimera (methyl-PROTAC). The methyl-PROTAC incorporates a methylated cytosine into an oligodeoxynucleotide moiety to recruit MeCP2 for targeted degradation in a von Hippel-Lindau- and proteasome-dependent manner, thus displaying antiproliferative effects in cancer cells reliant on MeCP2 overexpression. This selective cytotoxicity endows methyl-PROTAC with the capacity to selectively eliminate cancer cells that are addicted to the overexpression of the MeCP2 oncoprotein. Furthermore, methyl-PROTAC-mediated MeCP2 degradation induces apoptosis in cancer cells. These findings underscore the therapeutic potential of methyl-PROTAC to degrade undruggable epigenetic regulatory proteins. In summary, the development of methyl-PROTAC introduces an innovative strategy by designing a modified nucleotide-based degradation approach for manipulating epigenetic factors, thereby representing a promising avenue for the advancement of PROTAC-based therapeutics.
中文翻译:

基于甲基化核苷酸的蛋白水解靶向嵌合体能够靶向降解甲基-CpG 结合蛋白 2
甲基 CpG 结合蛋白 2 (MeCP2) 是 DNA 甲基化的解读器,因其在神经系统和神经发育障碍中的功能而被广泛研究。新的证据表明 MeCP2 在癌症中发挥致癌作用;然而,开发 MeCP2 靶向疗法仍然是一个挑战。这项工作试图通过引入一种基于甲基化核苷酸的靶向嵌合体来解决这个问题,称为甲基蛋白水解靶向嵌合体(甲基-PROTAC)。甲基-PROTAC将甲基化胞嘧啶整合到寡脱氧核苷酸部分中,以招募MeCP2以von Hippel-Lindau和蛋白酶体依赖性方式进行靶向降解,从而在依赖MeCP2过表达的癌细胞中显示出抗增殖作用。这种选择性细胞毒性赋予甲基-PROTAC 选择性消除过度表达 MeCP2 癌蛋白的癌细胞的能力。此外,甲基 PROTAC 介导的 MeCP2 降解会诱导癌细胞凋亡。这些发现强调了甲基-PROTAC 降解不可成药的表观遗传调节蛋白的治疗潜力。总之,甲基-PROTAC的开发引入了一种创新策略,通过设计一种改良的基于核苷酸的降解方法来操纵表观遗传因素,从而为基于PROTAC的治疗的发展提供了一条有希望的途径。
更新日期:2023-09-29
中文翻译:

基于甲基化核苷酸的蛋白水解靶向嵌合体能够靶向降解甲基-CpG 结合蛋白 2
甲基 CpG 结合蛋白 2 (MeCP2) 是 DNA 甲基化的解读器,因其在神经系统和神经发育障碍中的功能而被广泛研究。新的证据表明 MeCP2 在癌症中发挥致癌作用;然而,开发 MeCP2 靶向疗法仍然是一个挑战。这项工作试图通过引入一种基于甲基化核苷酸的靶向嵌合体来解决这个问题,称为甲基蛋白水解靶向嵌合体(甲基-PROTAC)。甲基-PROTAC将甲基化胞嘧啶整合到寡脱氧核苷酸部分中,以招募MeCP2以von Hippel-Lindau和蛋白酶体依赖性方式进行靶向降解,从而在依赖MeCP2过表达的癌细胞中显示出抗增殖作用。这种选择性细胞毒性赋予甲基-PROTAC 选择性消除过度表达 MeCP2 癌蛋白的癌细胞的能力。此外,甲基 PROTAC 介导的 MeCP2 降解会诱导癌细胞凋亡。这些发现强调了甲基-PROTAC 降解不可成药的表观遗传调节蛋白的治疗潜力。总之,甲基-PROTAC的开发引入了一种创新策略,通过设计一种改良的基于核苷酸的降解方法来操纵表观遗传因素,从而为基于PROTAC的治疗的发展提供了一条有希望的途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号