当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Oxidation of Amines to Hydroxylamines
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-09-29 , DOI: 10.1021/jacs.3c09172 Gang Wang 1, 2 , Tian Chen 1 , Kuiyong Jia 1 , Wencheng Ma 1 , Chen-Ho Tung 1 , Lei Liu 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-09-29 , DOI: 10.1021/jacs.3c09172 Gang Wang 1, 2 , Tian Chen 1 , Kuiyong Jia 1 , Wencheng Ma 1 , Chen-Ho Tung 1 , Lei Liu 1, 3
Affiliation
|
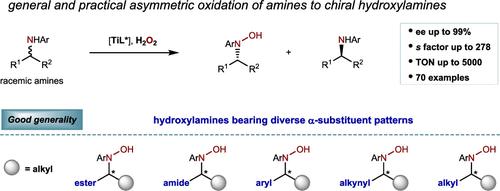
Chiral hydroxylamines are increasingly common structural elements in pharmaceuticals and agrochemicals, but their asymmetric synthesis remains challenging. Although enantioselective oxidation is the most straightforward method to prepare chiral oxides with a higher oxidation state, asymmetric and even nonasymmetric amine oxidation to hydroxylamines has been poorly addressed. We report a titanium-catalyzed asymmetric oxidation of racemic amines providing a broad range of structurally diverse chiral hydroxylamines with excellent chemo- and enantioselectivity. Notably, hydroxylamines bearing diverse substituent patterns on the stereocenters, including α,α-ester-alkyl, α,α-amide-alkyl, α,α-aryl-alkyl, α,α-alkynyl-alkyl, and α,α-dialkyl, are well tolerated with good functional group compatibility. Catalyst turnover numbers up to 5000 and selectivity factors up to 278 are observed. This finding offers a democratized platform to chiral hydroxylamines as design elements for drug discovery and provides insights into metal-catalyzed asymmetric oxidation of challenging substrates.
中文翻译:

胺催化不对称氧化成羟胺
手性羟胺是药物和农用化学品中越来越常见的结构元素,但其不对称合成仍然具有挑战性。尽管对映选择性氧化是制备具有较高氧化态的手性氧化物的最直接方法,但不对称甚至非不对称胺氧化成羟胺的问题尚未得到很好的解决。我们报道了钛催化的外消旋胺的不对称氧化,提供了多种结构多样的手性羟胺,具有优异的化学和对映选择性。值得注意的是,羟胺在立构中心上具有不同的取代基模式,包括α,α-酯-烷基、α,α-酰胺-烷基、α,α-芳基-烷基、α,α-炔基-烷基和α,α-二烷基,具有良好的耐受性和良好的官能团相容性。观察到催化剂周转次数高达 5000,选择性因子高达 278。这一发现为手性羟胺作为药物发现的设计元素提供了一个民主化平台,并为具有挑战性的底物的金属催化不对称氧化提供了见解。
更新日期:2023-09-29
中文翻译:

胺催化不对称氧化成羟胺
手性羟胺是药物和农用化学品中越来越常见的结构元素,但其不对称合成仍然具有挑战性。尽管对映选择性氧化是制备具有较高氧化态的手性氧化物的最直接方法,但不对称甚至非不对称胺氧化成羟胺的问题尚未得到很好的解决。我们报道了钛催化的外消旋胺的不对称氧化,提供了多种结构多样的手性羟胺,具有优异的化学和对映选择性。值得注意的是,羟胺在立构中心上具有不同的取代基模式,包括α,α-酯-烷基、α,α-酰胺-烷基、α,α-芳基-烷基、α,α-炔基-烷基和α,α-二烷基,具有良好的耐受性和良好的官能团相容性。观察到催化剂周转次数高达 5000,选择性因子高达 278。这一发现为手性羟胺作为药物发现的设计元素提供了一个民主化平台,并为具有挑战性的底物的金属催化不对称氧化提供了见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号