当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Study of the Epoxidation Intensification of Methyl Oleate by Phase-Transfer Catalysis in a Micellar Medium
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2023-09-27 , DOI: 10.1021/acs.iecr.3c01761
Michael Jabbour 1 , Imed Ben Talouba 1 , Laurent Balland 1 , Nordine Mouhab 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2023-09-27 , DOI: 10.1021/acs.iecr.3c01761
Michael Jabbour 1 , Imed Ben Talouba 1 , Laurent Balland 1 , Nordine Mouhab 1
Affiliation
|
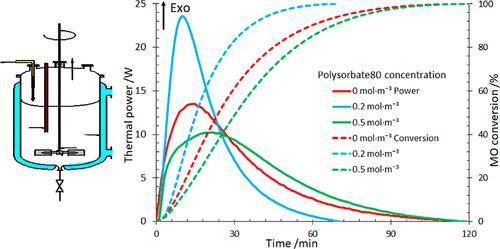
The aim of this work is to investigate the potential combination of phase-transfer catalysis (PTC) and microemulsions for the rapid epoxidation reaction of the double bonds present in methyl oleate, a monounsaturated vegetable oil. The reaction takes place in the presence of a quaternary ammonium salt, hydrogen peroxide, sodium tungstate, and phosphoric acid without solvents to which a nonionic surfactant is added. Two surfactants were tested: polysorbate 80 and Triton X-100. The results show that the coupling of PTC and micellar catalysis (MC) has reduced the reaction time by almost half of the epoxidation by PTC in the absence of surfactants. This intensification can be attributed to the behavior of the reaction medium formed by micelles playing the role of nanoreactors. The estimation of mass-transfer and chemical kinetic parameters was performed using the thermal power profile measured in a calorimetric reactor RC1-RTCal. Mass balance is evaluated using the main epoxidation reaction and that of the catalyst regeneration, whereas the mass-transfer kinetics between phases is determined by using a double film model. The activation energy of the epoxidation reaction was found to be 44 kJ·mol–1. Safety parameters, maximum temperature of synthesis reaction, and TD24 (temperature at which time to maximum rate is 24 h) were also determined, and their corresponding results demonstrated that this epoxidation process remains thermally safe.
中文翻译:

胶束介质中相转移催化油酸甲酯环氧化强化的动力学研究
这项工作的目的是研究相转移催化 (PTC) 和微乳液的潜在组合,用于单不饱和植物油油酸甲酯中双键的快速环氧化反应。该反应在季铵盐、过氧化氢、钨酸钠和磷酸存在下进行,无需添加非离子表面活性剂的溶剂。测试了两种表面活性剂:聚山梨酯 80 和 Triton X-100。结果表明,PTC 与胶束催化 (MC) 的偶联使 PTC 环氧化反应的反应时间缩短了几乎一半(在没有表面活性剂的情况下)。这种强化可归因于胶束形成的反应介质发挥纳米反应器的作用。使用量热反应器 RC1-RTCal 中测量的热功率曲线来估计传质和化学动力学参数。使用主要环氧化反应和催化剂再生反应来评估质量平衡,而相之间的传质动力学则通过使用双膜模型来确定。环氧化反应的活化能为44 kJ·mol –1。还确定了安全参数、合成反应的最高温度和T D 24 (达到最大速率的时间为24小时时的温度),相应的结果表明该环氧化过程保持热安全。
更新日期:2023-09-27
中文翻译:

胶束介质中相转移催化油酸甲酯环氧化强化的动力学研究
这项工作的目的是研究相转移催化 (PTC) 和微乳液的潜在组合,用于单不饱和植物油油酸甲酯中双键的快速环氧化反应。该反应在季铵盐、过氧化氢、钨酸钠和磷酸存在下进行,无需添加非离子表面活性剂的溶剂。测试了两种表面活性剂:聚山梨酯 80 和 Triton X-100。结果表明,PTC 与胶束催化 (MC) 的偶联使 PTC 环氧化反应的反应时间缩短了几乎一半(在没有表面活性剂的情况下)。这种强化可归因于胶束形成的反应介质发挥纳米反应器的作用。使用量热反应器 RC1-RTCal 中测量的热功率曲线来估计传质和化学动力学参数。使用主要环氧化反应和催化剂再生反应来评估质量平衡,而相之间的传质动力学则通过使用双膜模型来确定。环氧化反应的活化能为44 kJ·mol –1。还确定了安全参数、合成反应的最高温度和T D 24 (达到最大速率的时间为24小时时的温度),相应的结果表明该环氧化过程保持热安全。

































 京公网安备 11010802027423号
京公网安备 11010802027423号