当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Open-Microcolumn Array: A Novel Approach for Enhanced Electrocatalytic Bubble Desorption in Microreactors
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-09-28 , DOI: 10.1021/acsami.3c09901 Yibing Ma 1, 2 , Yaya Zhou 1, 2 , Yaqing Xie 1, 2 , Ningxuan Jin 1, 2 , Yushuang Cui 1, 2 , Yiqiang Qin 1, 2 , Haixiong Ge 1, 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-09-28 , DOI: 10.1021/acsami.3c09901 Yibing Ma 1, 2 , Yaya Zhou 1, 2 , Yaqing Xie 1, 2 , Ningxuan Jin 1, 2 , Yushuang Cui 1, 2 , Yiqiang Qin 1, 2 , Haixiong Ge 1, 2
Affiliation
|
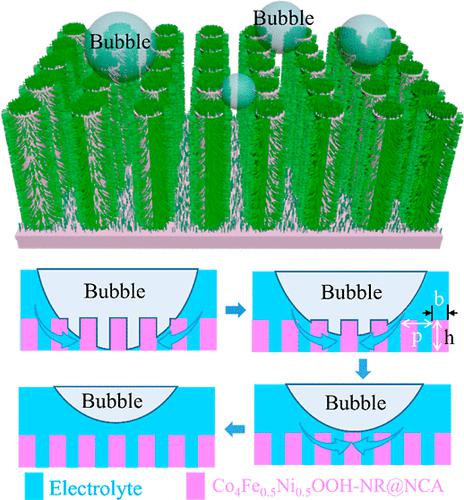
High-efficiency electrocatalytic water splitting requires high intrinsic activity of catalysts and even more importantly favorable mass transfer. However, gas bubbles adhering to the surface of catalysts limit the re-expose of catalytic active sites to the electrolyte and reduce the catalytic activities. The efficient desorption of bubbles can be facilitated by a hierarchical multiscale structure of the electrode surface. Herein, we report an opened periodic three-dimensional electrode composed of iron (Fe)-cobalt (Co)-nickel (Ni) (oxy)hydroxide nanorods (NRs) grown in situ on a high aspect ratio nickel microcolumn array (NCA) for electrocatalytic water splitting. Compared with the flat nickel plate, the NCA not only increases the surface area for catalyst loading but also improves the wettability of the electrolyte on the electrode surface, exhibiting superhydrophilicity/superaerophobicity (the electrolyte and the bubble contact angles were about ∼0 and 163°, respectively), which accelerates the bubble evolution and desorption process. The X-ray photoelectron spectroscopy indicates that the synergy of Fe–Co–Ni could enhance the ratio of Co3+/Co2+ and Ni3+/Ni2+ and promote the electrocatalytic activity. Benefiting from the microstructure design and synergistic effects, the Co4Fe0.5Ni0.5OOH-NR@NCA electrode achieves a superior OER performance with an overpotential of 199 mV at 10 mA·cm–2.
中文翻译:

开放式微柱阵列:微反应器中增强电催化气泡解吸的新方法
高效电催化水分解需要催化剂具有高的固有活性,更重要的是有利的传质。然而,附着在催化剂表面的气泡限制了催化活性位点再次暴露于电解质并降低了催化活性。电极表面的分层多尺度结构可以促进气泡的有效解吸。在此,我们报道了一种由铁(Fe)-钴(Co)-镍(Ni)(氧)氢氧化物纳米棒(NR)组成的开放式周期性三维电极,其在高纵横比镍微柱阵列(NCA)上原位生长电催化水分解。与平镍板相比,NCA不仅增加了催化剂负载的表面积,而且提高了电解液在电极表面的润湿性,表现出超亲水/超疏气性(电解液与气泡的接触角约为∼0和163°) ,分别),这加速了气泡的演化和解吸过程。X射线光电子能谱表明,Fe-Co-Ni的协同作用可以提高Co 3+ /Co 2+和Ni 3+ /Ni 2+的比例,提高电催化活性。得益于微观结构设计和协同效应,Co 4 Fe 0.5 Ni 0.5 OOH-NR@NCA电极实现了优异的OER性能,在10 mA·cm –2下的过电势为199 mV 。
更新日期:2023-09-28
中文翻译:

开放式微柱阵列:微反应器中增强电催化气泡解吸的新方法
高效电催化水分解需要催化剂具有高的固有活性,更重要的是有利的传质。然而,附着在催化剂表面的气泡限制了催化活性位点再次暴露于电解质并降低了催化活性。电极表面的分层多尺度结构可以促进气泡的有效解吸。在此,我们报道了一种由铁(Fe)-钴(Co)-镍(Ni)(氧)氢氧化物纳米棒(NR)组成的开放式周期性三维电极,其在高纵横比镍微柱阵列(NCA)上原位生长电催化水分解。与平镍板相比,NCA不仅增加了催化剂负载的表面积,而且提高了电解液在电极表面的润湿性,表现出超亲水/超疏气性(电解液与气泡的接触角约为∼0和163°) ,分别),这加速了气泡的演化和解吸过程。X射线光电子能谱表明,Fe-Co-Ni的协同作用可以提高Co 3+ /Co 2+和Ni 3+ /Ni 2+的比例,提高电催化活性。得益于微观结构设计和协同效应,Co 4 Fe 0.5 Ni 0.5 OOH-NR@NCA电极实现了优异的OER性能,在10 mA·cm –2下的过电势为199 mV 。































 京公网安备 11010802027423号
京公网安备 11010802027423号