Science of the Total Environment ( IF 8.2 ) Pub Date : 2023-09-27 , DOI: 10.1016/j.scitotenv.2023.167427 Danni Zhang 1 , Yuting Jin 2 , Yumeng Wang 3 , Shaofeng Wang 4 , Fan Xiao 5 , Ying Wang 6 , Duo Wang 7 , Dake Xu 2 , Fuhui Wang 2 , Yongfeng Jia 8
|
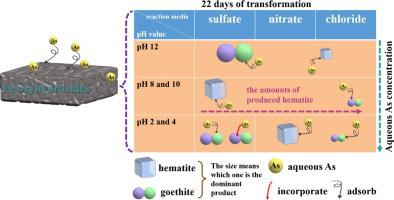
Understanding the nature of arsenic (As) adsorbed on FeIII oxyhydroxides, and the subsequent behavior of As during the crystallization process, is critical to predicting its fate in a range of natural and engineered settings. In this work, As adsorbed on FeIII oxyhydroxides formed in the different reaction media at different pH values were characterized with X-ray diffraction (XRD), Raman spectra, transmission electron microscopy (TEM), and extended X-ray absorption fine structure spectroscopy (EXAFS) to determine how As is redistributed during the crystallization process. Results showed that at pH 12, a quarter of the added As was still left in the liquid phase with the formation of goethite and hematite as the major and minor product. The concentration of As was found to be the lowest at pH 4 which is independent of the reaction media, indicating the importance of pH value in the crystallization process of the As adsorbed FeIII oxyhydroxides. Under acidic conditions, sulfate and chloride media favored the formation of goethite and hematite, respectively. Arsenic can indeed be incorporated into the structure of the formed goethite at pH 4. The morphology of the formed products changed to rhombus-like particles if both goethite and hematite appeared as the later as the dominant product.
中文翻译:

FeIII 羟基氧化物结晶过程中砷的归宿:反应介质、pH 值和相对较低砷负载下 Fe/As 摩尔比的影响
了解吸附在 Fe III羟基氧化物上的砷 (As) 的性质,以及 As 在结晶过程中的后续行为,对于预测其在一系列自然和工程环境中的命运至关重要。在这项工作中,利用 X 射线衍射 (XRD)、拉曼光谱、透射电子显微镜 (TEM) 和扩展 X 射线吸收精细结构光谱对在不同 pH 值下的不同反应介质中形成的 Fe III羟基氧化物上吸附的 As 进行了表征(EXAFS) 以确定 As 在结晶过程中如何重新分布。结果表明,在 pH 12 时,添加的砷的四分之一仍留在液相中,并形成针铁矿和赤铁矿作为主要和次要产物。发现在 pH 4 时 As 的浓度最低,这与反应介质无关,表明 pH 值在 As 吸附的 Fe III羟基氧化物的结晶过程中的重要性。在酸性条件下,硫酸盐和氯化物介质分别有利于针铁矿和赤铁矿的形成。在pH 4 时,砷确实可以结合到形成的针铁矿的结构中。如果针铁矿和赤铁矿都作为后者作为主要产物出现,则形成的产物的形态变为菱形颗粒。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号