Matter ( IF 17.3 ) Pub Date : 2023-09-28 , DOI: 10.1016/j.matt.2023.09.003 Jinchang Fan , Yunlong Zhang , Wei Liu , Mingrun Li , Yafeng Cai , Qinqin Ji , Zhenchao Zhao , Guangjin Hou , Aowen Li , Wu Zhou , Liang Yu , Dehui Deng
|
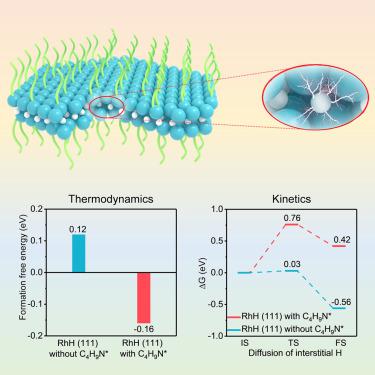
Transition metal hydrides hold great potential as high-efficiency catalysts for hydrogen-involving reactions, benefiting from finely modulable electronic states and reaction paths by interstitial hydrogen. But their severe thermodynamic instability brings challenges for their preparation and utilization under ambient conditions. Herein, we report a ligand confinement effect to break thermodynamic restriction and for the first time realize the construction of ambient-stable two-dimensional (2D) rhodium hydride (RhH) nanosheets. By using electron-attracting n-butylamino ligands to enhance the interaction between Rh and interstitial hydrogen, the formation of 2D RhH becomes thermodynamically permissible. It exhibits a superior hydrogen evolution reaction (HER) activity with a lower overpotential and 3.1 times and 1.4 times higher mass activity than 2D Rh catalyst and commercial Pt/C catalyst, respectively. Theoretical calculations reveal the significant effect of interstitial H atoms and surface ligands in boosting the HER activity of Rh, by moderately weakening hydrogen adsorption and accelerating H2 desorption via a ligand-mediated H-transfer mechanism.
中文翻译:

配体限制的二维氢化铑促进析氢
受益于间隙氢可精细调节的电子态和反应路径,过渡金属氢化物作为涉及氢的反应的高效催化剂具有巨大的潜力。但它们严重的热力学不稳定性给它们在环境条件下的制备和使用带来了挑战。在此,我们报告了配体限制效应,打破了热力学限制,并首次实现了环境稳定的二维(2D)氢化铑(RhH)纳米片的构建。通过使用吸电子正丁基氨基配体增强Rh与间隙氢之间的相互作用,热力学上允许形成2D RhH。它表现出优异的析氢反应(HER)活性,具有较低的过电势,质量活性分别比 2D Rh 催化剂和商用 Pt/C 催化剂高 3.1 倍和 1.4 倍。理论计算揭示了间隙H原子和表面配体通过配体介导的H转移机制适度减弱氢吸附并加速H 2解吸,从而对提高Rh的HER活性具有显着影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号