当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective formal alkenylation of β,β-disubstituted enesulfinamides for constructing 1,5- and 1,4-dicarbonyl derivatives bearing less-accessible acyclic α-quaternary stereocenters
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2023-09-28 , DOI: 10.1039/d3qo01381b Chong-Lin Zhu 1, 2 , Chong-Dao Lu 1, 3
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2023-09-28 , DOI: 10.1039/d3qo01381b Chong-Lin Zhu 1, 2 , Chong-Dao Lu 1, 3
Affiliation
|
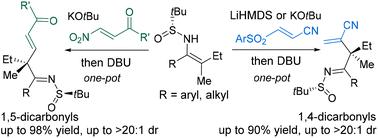
Geometry-defined β,β-disubstituted enesulfinamides undergo one-pot conjugate addition–elimination cascade to afford α-alkenylated ketimines with high stereocontrol. By tuning the substituents on Michael acceptors, formal alkenylation transformation allows regiodivergent construction of 1,5- and 1,4-dicarbonyl derivatives bearing less-accessible acyclic quaternary α-stereocenters substituted with two sterically and electronically similar groups (e.g., methyl and ethyl).
中文翻译:

β,β-二取代烯亚磺酰胺的立体选择性形式烯基化,用于构建带有不易接近的无环 α-四元立构中心的 1,5- 和 1,4-二羰基衍生物
几何定义的 β,β-二取代烯亚磺酰胺经过一锅共轭加成-消除级联,得到具有高度立体控制的 α-烯基化酮亚胺。通过调整迈克尔受体上的取代基,正式的烯基化转化允许区域发散地构建带有不易接近的无环四元α-立构中心的1,5-和1,4-二羰基衍生物,并被两个空间和电子相似的基团(例如甲基和乙基)取代。
更新日期:2023-09-28
中文翻译:

β,β-二取代烯亚磺酰胺的立体选择性形式烯基化,用于构建带有不易接近的无环 α-四元立构中心的 1,5- 和 1,4-二羰基衍生物
几何定义的 β,β-二取代烯亚磺酰胺经过一锅共轭加成-消除级联,得到具有高度立体控制的 α-烯基化酮亚胺。通过调整迈克尔受体上的取代基,正式的烯基化转化允许区域发散地构建带有不易接近的无环四元α-立构中心的1,5-和1,4-二羰基衍生物,并被两个空间和电子相似的基团(例如甲基和乙基)取代。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号