Journal of Allergy and Clinical Immunology ( IF 11.4 ) Pub Date : 2023-09-26 , DOI: 10.1016/j.jaci.2023.08.036
Kathleen Baysac 1 , Guangping Sun 2 , Hiroto Nakano 1 , Elizabeth G Schmitz 1 , Anthony C Cruz 1 , Charles Fisher 1 , Alexis C Bailey 1 , , Emily Mace 3 , Joshua D Milner 3 , Michael J Ombrello 1
|
Background
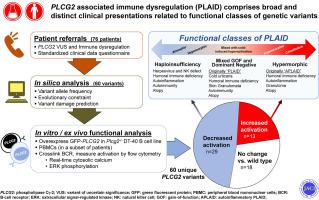
Pathogenic variants of phospholipase C gamma 2 (PLCG2) cause 2 related forms of autosomal-dominant immune dysregulation (ID), PLCγ2-associated antibody deficiency and immune dysregulation (PLAID) and autoinflammatory PLAID (APLAID). Since describing these conditions, many PLCG2 variants of uncertain significance have been identified by clinical sequencing of patients with diverse features of ID.
Objective
We sought to functionally classify PLCG2 variants and explore known and novel genotype-function-phenotype relationships.
Methods
Clinical data from patients with PLCG2 variants were obtained via standardized questionnaire. PLCG2 variants were generated by mutagenesis of enhanced green fluorescent protein (EGFP)-PLCG2 plasmid, which was overexpressed in Plcg2-deficient DT-40 B cells. B-cell receptor–induced calcium flux and extracellular signal-regulated kinase phosphorylation were assayed by flow cytometry. In some cases, stimulation-induced calcium flux was also measured in primary patient cells.
Results
Three-fourths of PLCG2 variants produced functional alteration of B-cell activation, in vitro. Thirteen variants led to gain of function (GOF); however, most functional variants defined a new class of PLCG2 mutation, monoallelic loss of function (LOF). Susceptibility to infection and autoinflammation were common with both GOF and LOF variants, whereas a new phenotypic cluster consisting of humoral immune deficiency, autoinflammation, susceptibility to herpesvirus infection, and natural killer cell dysfunction was observed in association with multiple heterozygous LOF variants detected in both familial and sporadic cases. In some cases, PLCG2 variants produced greater effects in natural killer cells than in B cells.
Conclusions
This work expands the genotypic and phenotypic associations with functional variation in PLCG2, including a novel form of ID in carriers of heterozygous loss of PLCG2 function. It also demonstrates the need for more diverse assays for assessing the impact of PLCG2 variants on human disease.
中文翻译:

PLCG2 相关免疫失调 (PLAID) 包括与遗传变异功能类别相关的广泛而不同的临床表现
背景
磷脂酶 C γ 2 (PLCG2) 的致病性变异导致 2 种相关形式的常染色体显性遗传免疫失调 (ID)、PLCγ2 相关抗体缺陷和免疫失调 (PLAID) 以及自身炎症性 PLAID (APLAID)。自描述这些情况以来,通过对具有不同 ID 特征的患者进行临床测序,已经确定了许多意义不明的 PLCG2 变异。
目的
我们试图对 PLCG2 变体进行功能分类,并探索已知和新的基因型 - 功能 - 表型关系。
方法
通过标准化问卷获得 PLCG2 变异患者的临床资料。PLCG2 变体是通过增强绿色荧光蛋白 (EGFP)-PLCG2 质粒的诱变产生的,该质粒在 Plcg2 缺陷型 DT-40 B 细胞中过表达。通过流式细胞术测定 B 细胞受体诱导的钙通量和细胞外信号调节的激酶磷酸化。在某些情况下,还在原代患者细胞中测量了刺激诱导的钙通量。
结果
四分之三的 PLCG2 变体在 体外产生 B 细胞活化的功能改变。13 种变异导致功能获得 (GOF);然而,大多数功能变异定义了一类新的 PLCG2 突变,即单等位基因功能丧失 (LOF)。对感染和自身炎症的易感性在 GOF 和 LOF 变体中都很常见,而观察到由体液免疫缺陷、自身炎症、疱疹病毒感染易感性和自然杀伤细胞功能障碍组成的新表型簇与在家族性和散发病例中检测到的多个杂合 LOF 变体相关。在某些情况下,PLCG2 变体在自然杀伤细胞中产生的影响大于在 B 细胞中。
结论
这项工作扩展了与 PLCG2 功能变异的基因型和表型关联,包括 PLCG2 功能杂合缺失载体中的一种新型 ID。它还表明需要更多样化的检测方法来评估 PLCG2 变体对人类疾病的影响。































 京公网安备 11010802027423号
京公网安备 11010802027423号