当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Salt-Dependent Phase Re-entry of Weak Polyelectrolyte Complexes: from Associative to Segregative Liquid–Liquid Phase Separation
Macromolecules ( IF 5.1 ) Pub Date : 2023-09-26 , DOI: 10.1021/acs.macromol.3c01468 Huiling Li 1 , Ying Liu 1 , Fujie Lan 2 , Mohsen Ghasemi 2 , Ronald G. Larson 1, 2
Macromolecules ( IF 5.1 ) Pub Date : 2023-09-26 , DOI: 10.1021/acs.macromol.3c01468 Huiling Li 1 , Ying Liu 1 , Fujie Lan 2 , Mohsen Ghasemi 2 , Ronald G. Larson 1, 2
Affiliation
|
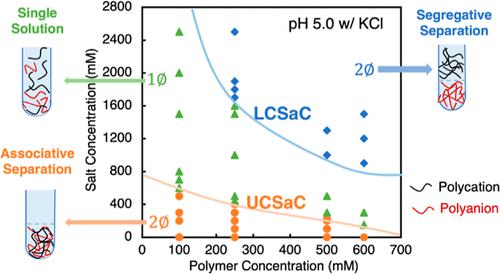
A high-salt phase-separation re-entry is observed in mixtures of poly(diallyldimethylammonium chloride), a strong polycation, and poly(acrylic acid) (PAA), a partially charged polyanion, within the pH range 4.7–5.3. This intriguing phenomenon exclusively occurs at salt concentrations exceeding the critical salt concentration required for dissolving the coacervate formed at low salt concentrations, here named the “upper critical salt concentration”, and at monomer concentrations exceeding 0.1 M for each polymer. The transition from associative phase separation at low salt concentrations to a single solution and ultimately to segregative separation at high salt concentrations, called the “lower critical salt concentration”, arises from the interplay between electrostatic interactions and the hydrophobicity of neutral PAA monomers in a high-salt solvent. To explain this transition, we use a theory combining short-range ion pairing and counterion condensation with long-range electrostatics using the random phase approximation and with hydrophobic interactions between PAA neutral monomers and water. The latter is modeled through a Flory–Huggins χ parameter of around 0.6. Literature observations of a continuous transition from associative to segregative phase transition with increasing salt concentration, without a homogeneous single-phase solution at intermediate salt concentration, are also predicted and discussed.
中文翻译:

弱聚电解质复合物的盐依赖性相再进入:从缔合到分离液-液相分离
在聚(二烯丙基二甲基氯化铵)(一种强聚阳离子)和聚丙烯酸(PAA)(一种部分带电的聚阴离子)的混合物中,在 pH 范围 4.7-5.3 内观察到高盐相分离再进入。这种有趣的现象仅发生在盐浓度超过溶解在低盐浓度下形成的凝聚层所需的临界盐浓度(这里称为“上临界盐浓度”)以及每种聚合物的单体浓度超过0.1M时。从低盐浓度下的缔合相分离到单一溶液并最终到高盐浓度下的分离分离的转变,称为“下临界盐浓度”,是由高盐浓度下静电相互作用和中性 PAA 单体的疏水性之间的相互作用引起的。 -盐溶剂。为了解释这种转变,我们使用了一种理论,将短程离子配对和抗衡离子缩合与使用随机相近似的长程静电以及 PAA 中性单体和水之间的疏水相互作用相结合。后者通过大约 0.6 的 Flory–Huggins χ 参数进行建模。还预测和讨论了文献观察到随着盐浓度的增加,从缔合相变到分离相变的连续转变,而在中间盐浓度下没有均匀的单相溶液。
更新日期:2023-09-26
中文翻译:

弱聚电解质复合物的盐依赖性相再进入:从缔合到分离液-液相分离
在聚(二烯丙基二甲基氯化铵)(一种强聚阳离子)和聚丙烯酸(PAA)(一种部分带电的聚阴离子)的混合物中,在 pH 范围 4.7-5.3 内观察到高盐相分离再进入。这种有趣的现象仅发生在盐浓度超过溶解在低盐浓度下形成的凝聚层所需的临界盐浓度(这里称为“上临界盐浓度”)以及每种聚合物的单体浓度超过0.1M时。从低盐浓度下的缔合相分离到单一溶液并最终到高盐浓度下的分离分离的转变,称为“下临界盐浓度”,是由高盐浓度下静电相互作用和中性 PAA 单体的疏水性之间的相互作用引起的。 -盐溶剂。为了解释这种转变,我们使用了一种理论,将短程离子配对和抗衡离子缩合与使用随机相近似的长程静电以及 PAA 中性单体和水之间的疏水相互作用相结合。后者通过大约 0.6 的 Flory–Huggins χ 参数进行建模。还预测和讨论了文献观察到随着盐浓度的增加,从缔合相变到分离相变的连续转变,而在中间盐浓度下没有均匀的单相溶液。































 京公网安备 11010802027423号
京公网安备 11010802027423号