当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitrene C−H Bond Insertion Approach to Carbazolones and Indolones, and a Reactivity Departure for 7-Membered Analogues**
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2023-09-26 , DOI: 10.1002/chem.202302995
Mahesh K Lakshman 1, 2 , Dellamol Sebastian 1, 2 , Padmanava Pradhan 1 , Michelle C Neary 3 , Alexis M Piette 4 , Samuel P Trzebiatowski 4 , Alexander E K Henriques 4 , Patrick H Willoughby 4
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2023-09-26 , DOI: 10.1002/chem.202302995
Mahesh K Lakshman 1, 2 , Dellamol Sebastian 1, 2 , Padmanava Pradhan 1 , Michelle C Neary 3 , Alexis M Piette 4 , Samuel P Trzebiatowski 4 , Alexander E K Henriques 4 , Patrick H Willoughby 4
Affiliation
|
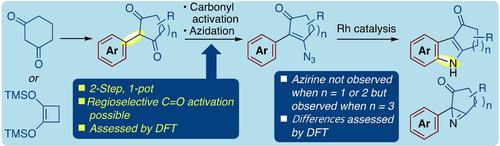
2-Arylcyclohexane-1,3-diones and 2-arylcyclopentane-1,3-diones can be converted in a facile 2-step, 1-pot manner to 2-aryl-3-azidocycloalk-2-en-1-ones. These azides can be smoothly cyclized to carbazolones and indolones with catalytic Rh2(O2CC7H15)4. Although 3-azido-2-phenylcyclohept-2-en-1-one could be readily prepared from 2-phenylcycloheptane-1,3-dione, by contrast the Rh-catalyzed cyclization gave an azirine in preference to the indole. Mechanistic and DFT studies complement the synthetic work.
中文翻译:

咔唑酮和吲哚酮的氮烯 C−H 键插入方法以及 7 元类似物的反应性偏离**
2-芳基环己烷-1,3-二酮和2-芳基环戊烷-1,3-二酮可以通过简单的两步、一锅法转化为2-芳基-3-叠氮基环烷-2-en-1-酮。这些叠氮化物可以在催化Rh 2 (O 2 CC 7 H 15 ) 4的作用下顺利环化为咔唑酮和吲哚酮。虽然 3-叠氮基-2-苯基环庚-2-en-1-酮可以很容易地从 2-苯基环庚烷-1,3-二酮制备,但相比之下,Rh 催化的环化反应优先于吲哚生成氮丙啶。机理和 DFT 研究补充了综合工作。
更新日期:2023-09-26
中文翻译:

咔唑酮和吲哚酮的氮烯 C−H 键插入方法以及 7 元类似物的反应性偏离**
2-芳基环己烷-1,3-二酮和2-芳基环戊烷-1,3-二酮可以通过简单的两步、一锅法转化为2-芳基-3-叠氮基环烷-2-en-1-酮。这些叠氮化物可以在催化Rh 2 (O 2 CC 7 H 15 ) 4的作用下顺利环化为咔唑酮和吲哚酮。虽然 3-叠氮基-2-苯基环庚-2-en-1-酮可以很容易地从 2-苯基环庚烷-1,3-二酮制备,但相比之下,Rh 催化的环化反应优先于吲哚生成氮丙啶。机理和 DFT 研究补充了综合工作。

































 京公网安备 11010802027423号
京公网安备 11010802027423号