当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective and Robust Ru Catalyst for the Aqueous Phase Aerobic Oxidation of Furfural to 2-Furoic Acid
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-09-26 , DOI: 10.1021/acsami.3c09965 Priya Lokhande 1, 2 , Paresh L Dhepe 1, 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-09-26 , DOI: 10.1021/acsami.3c09965 Priya Lokhande 1, 2 , Paresh L Dhepe 1, 2
Affiliation
|
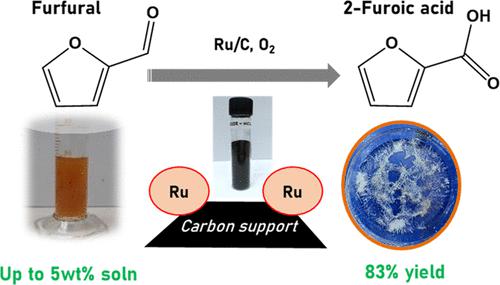
Synthesis of 2-furoic acid (FURA) via oxidation of furfural (FAL) is vital in evolving the biorefinery concept as FURA has numerous important applications in the pharmaceuticals and optic areas. Though few works on this reaction are done, those are marred with shortcomings such as the nonrecyclability of catalyst, dilute solutions, lower yields, or use of H2O2 as an oxidizing agent. Herein, we report catalytic aqueous phase oxidation of FAL to FURA using molecular oxygen as an oxidizing agent. For the synthesis of FURA, various catalysts with a combination of metal (Pt, Pd, Ru) and supports (carbon, Al2O3) were prepared and characterized by multiple techniques (X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), Brunauer–Emmett–Teller (BET), X-ray photoelectron spectroscopy (XPS)). Oxidation of FAL carried out over 5 wt % Ru/C catalyst in the presence of Na2CO3 yielded 83% of FURA at 120 °C and 15 bar oxygen pressure. The catalyst could show potential for reusability as similar activity was achieved after subjecting the spent catalyst to mild reduction treatment (150 °C). Studies on the effects of temperature, pressure, and time could help accomplish enhanced yields of FURA. Additionally, learning about the effect of base (weak/strong/solid) revealed that due to the weak basicity of Na2CO3, higher yields could be achieved by maintaining approximately a pH of 11, which is optimal for suppressing side reactions. Under the given conditions, FURA is stable (>90%) and also adsorption studies divulge that it is immediately removed from the catalyst surface, and hence higher yields could be achieved in our catalytic system. Using the initial rates methodology, an activation energy of 21.91 kJ mol–1 was derived and also a high turn over frequency (TOF) (85.9 h–1) was observed under optimized conditions.
中文翻译:

用于糠醛水相有氧氧化生成 2-糠酸的选择性鲁棒 Ru 催化剂
通过氧化糠醛 (FAL) 合成 2-糠酸 (FURA) 对于生物精炼概念的发展至关重要,因为 FURA 在制药和光学领域有许多重要的应用。尽管对该反应的研究很少,但这些反应都存在一些缺点,例如催化剂的不可回收性、稀溶液、较低的产率或使用H 2 O 2作为氧化剂。在此,我们报道了使用分子氧作为氧化剂将 FAL 催化水相氧化为 FURA。为了合成 FURA,制备了各种由金属(Pt、Pd、Ru)和载体(碳、Al 2 O 3 )组合而成的催化剂,并通过多种技术(X 射线衍射 (XRD)、扫描电子显微镜、 SEM)、透射电子显微镜(TEM)、Brunauer-Emmett-Teller(BET)、X 射线光电子能谱(XPS))。FAL 的氧化在 Na 2 CO 3存在下在 5 wt% Ru/C 催化剂上进行,在 120 °C 和 15 bar 氧气压力下产生 83% 的 FURA。该催化剂具有可重复使用的潜力,因为对废催化剂进行温和还原处理(150℃)后可达到类似的活性。对温度、压力和时间影响的研究有助于提高 FURA 的产量。此外,了解碱(弱/强/固体)的影响表明,由于Na 2 CO 3的弱碱性,通过维持大约11的pH值可以实现更高的产率,这对于抑制副反应是最佳的。在给定条件下,FURA 是稳定的 (>90%),而且吸附研究表明它会立即从催化剂表面去除,因此我们的催化系统可以实现更高的产率。使用初始速率方法,得出21.91 kJ mol –1的活化能,并且在优化条件下观察到高周转频率 (TOF) (85.9 h –1 )。
更新日期:2023-09-26
中文翻译:

用于糠醛水相有氧氧化生成 2-糠酸的选择性鲁棒 Ru 催化剂
通过氧化糠醛 (FAL) 合成 2-糠酸 (FURA) 对于生物精炼概念的发展至关重要,因为 FURA 在制药和光学领域有许多重要的应用。尽管对该反应的研究很少,但这些反应都存在一些缺点,例如催化剂的不可回收性、稀溶液、较低的产率或使用H 2 O 2作为氧化剂。在此,我们报道了使用分子氧作为氧化剂将 FAL 催化水相氧化为 FURA。为了合成 FURA,制备了各种由金属(Pt、Pd、Ru)和载体(碳、Al 2 O 3 )组合而成的催化剂,并通过多种技术(X 射线衍射 (XRD)、扫描电子显微镜、 SEM)、透射电子显微镜(TEM)、Brunauer-Emmett-Teller(BET)、X 射线光电子能谱(XPS))。FAL 的氧化在 Na 2 CO 3存在下在 5 wt% Ru/C 催化剂上进行,在 120 °C 和 15 bar 氧气压力下产生 83% 的 FURA。该催化剂具有可重复使用的潜力,因为对废催化剂进行温和还原处理(150℃)后可达到类似的活性。对温度、压力和时间影响的研究有助于提高 FURA 的产量。此外,了解碱(弱/强/固体)的影响表明,由于Na 2 CO 3的弱碱性,通过维持大约11的pH值可以实现更高的产率,这对于抑制副反应是最佳的。在给定条件下,FURA 是稳定的 (>90%),而且吸附研究表明它会立即从催化剂表面去除,因此我们的催化系统可以实现更高的产率。使用初始速率方法,得出21.91 kJ mol –1的活化能,并且在优化条件下观察到高周转频率 (TOF) (85.9 h –1 )。






































 京公网安备 11010802027423号
京公网安备 11010802027423号