Tetrahedron Letters ( IF 1.5 ) Pub Date : 2023-09-26 , DOI: 10.1016/j.tetlet.2023.154769 Manzoor Zaman , Muhammad Hasan , Anatoly A. Peshkov , Aleksandra Puzyk , Yuqing Wang , Chang-Keun Lim , Olga P. Pereshivko , Vsevolod A. Peshkov
|
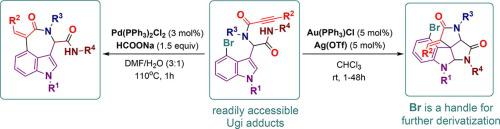
4-Bromo-1H-indole-3-carbaldehyde has been explored as a key building block for a post-Ugi assembly of azepino[3,4,5-cd]indoles and spiroindolines. First, the corresponding Ugi adducts were successfully employed in palladium-catalyzed reductive Heck cyclization producing a range of azepinoindoles. Then, 4-bromo-1H-indole-3-carbaldehyde-derived Ugi adducts were subjected to cationic gold catalysis affording tetracyclic spiroindolines. Furthermore, the aryl bromide moiety retained in the resulting spiroindolines was found to be amendable to further functionalization via Suzuki and Sonogashira couplings.
中文翻译:

4-溴-1H-吲哚-3-甲醛衍生的Ugi加合物的多样化:获得氮杂[3,4,5-cd]吲哚和螺吲哚啉
4-溴-1 H -吲哚-3-甲醛已被探索作为 azepino[3,4,5-cd]吲哚和螺吲哚啉的后 Ugi 组装的关键构建模块。首先,相应的Ugi加合物成功地用于钯催化的还原Heck环化反应中,产生一系列氮杂吲哚。然后,将4-溴-1H-吲哚-3-甲醛衍生的Ugi加合物进行阳离子金催化,得到四环螺吲哚啉。此外,发现所得螺二氢吲哚中保留的芳基溴部分可通过 Suzuki 和 Sonogashira 偶联进一步官能化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号