当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlled extracellular vesicles release from aminoguanidine nanoparticle-loaded polylysine hydrogel for synergistic treatment of spinal cord injury
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2023-09-21 , DOI: 10.1016/j.jconrel.2023.09.026 Shaoke Wang 1 , Rui Wang 2 , Jiangjie Chen 3 , Biao Yang 4 , Jiawei Shu 3 , Feng Cheng 3 , Yiqing Tao 3 , Kesi Shi 3 , Chenggui Wang 5 , Jingkai Wang 3 , Kaishun Xia 3 , Yuang Zhang 3 , Qixin Chen 3 , Chengzhen Liang 3 , Jianbin Tang 2 , Fangcai Li 3
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2023-09-21 , DOI: 10.1016/j.jconrel.2023.09.026 Shaoke Wang 1 , Rui Wang 2 , Jiangjie Chen 3 , Biao Yang 4 , Jiawei Shu 3 , Feng Cheng 3 , Yiqing Tao 3 , Kesi Shi 3 , Chenggui Wang 5 , Jingkai Wang 3 , Kaishun Xia 3 , Yuang Zhang 3 , Qixin Chen 3 , Chengzhen Liang 3 , Jianbin Tang 2 , Fangcai Li 3
Affiliation
|
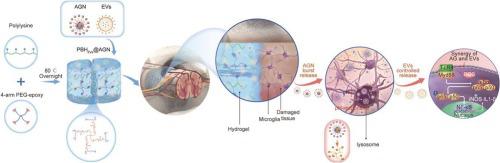
Pharmaceutical treatments are critical for the acute and subacute phases of spinal cord injury (SCI) and significantly impact patients' prognoses. However, there is a lack of a precise, multitemporal, integrated drug delivery system for medications administered in both phases. In this study, we prepare a hybrid polylysine-based hydrogel (PBH@AGN) comprising short-term release of pH-responsive aminoguanidine nanoparticles (AGN) and sustained release of extracellular vesicles (EVs) for synergistic SCI treatment. When AGN is exposed to the acidic environment at the injury site, it quickly diffuses out of the hydrogel and releases the majority of the aminoguanidine within 24 h, reducing oxidative stress in lesion tissues. Enriched EVs are gradually released from the hydrogel and remain in the tissue for weeks, providing a long-term anti-inflammatory effect and further ensuring axonal regeneration. Fast-releasing aminoguanidine can cooperate with slow-release EVs to treat SCI more effectively by reducing the production of proinflammatory cytokines and blocking the TLR4/Myd88/NF-κB inflammatory pathway, creating a sustained anti-inflammatory microenvironment for SCI recovery. Our in vivo experiments demonstrate that PBH@AGN reduces the occurrence of scar tissue, encourages remyelination, and speeds up axonal regeneration. Herein, this multi-drug delivery system, which combines the acute release of aminoguanidine and the sustained release of EVs is highly effective for synergistically managing the challenging pathological processes after SCI.
中文翻译:

负载氨基胍纳米颗粒的聚赖氨酸水凝胶控制细胞外囊泡的释放用于协同治疗脊髓损伤
药物治疗对于脊髓损伤 (SCI) 的急性期和亚急性期至关重要,并显着影响患者的预后。然而,对于这两个阶段的给药,缺乏精确的、多时相的、集成的药物输送系统。在这项研究中,我们制备了一种混合聚赖氨酸水凝胶(PBH@AGN),包含短期释放的pH响应性氨基胍纳米颗粒(AGN)和持续释放的细胞外囊泡(EV),用于协同SCI治疗。当AGN暴露于损伤部位的酸性环境时,它会迅速从水凝胶中扩散出来,并在24小时内释放出大部分氨基胍,从而减少病变组织的氧化应激。丰富的 EV 逐渐从水凝胶中释放出来,并在组织中保留数周,提供长期的抗炎作用,并进一步确保轴突再生。速释氨基胍可与缓释EVs配合,通过减少促炎细胞因子的产生、阻断TLR4/Myd88/NF-κB炎症通路,更有效地治疗SCI,为SCI恢复创造持续的抗炎微环境。我们的体内实验表明,PBH@AGN 减少了疤痕组织的发生,促进髓鞘再生,并加速轴突再生。这种多药物递送系统结合了氨基胍的急性释放和 EV 的持续释放,对于协同管理 SCI 后具有挑战性的病理过程非常有效。
更新日期:2023-09-21
中文翻译:

负载氨基胍纳米颗粒的聚赖氨酸水凝胶控制细胞外囊泡的释放用于协同治疗脊髓损伤
药物治疗对于脊髓损伤 (SCI) 的急性期和亚急性期至关重要,并显着影响患者的预后。然而,对于这两个阶段的给药,缺乏精确的、多时相的、集成的药物输送系统。在这项研究中,我们制备了一种混合聚赖氨酸水凝胶(PBH@AGN),包含短期释放的pH响应性氨基胍纳米颗粒(AGN)和持续释放的细胞外囊泡(EV),用于协同SCI治疗。当AGN暴露于损伤部位的酸性环境时,它会迅速从水凝胶中扩散出来,并在24小时内释放出大部分氨基胍,从而减少病变组织的氧化应激。丰富的 EV 逐渐从水凝胶中释放出来,并在组织中保留数周,提供长期的抗炎作用,并进一步确保轴突再生。速释氨基胍可与缓释EVs配合,通过减少促炎细胞因子的产生、阻断TLR4/Myd88/NF-κB炎症通路,更有效地治疗SCI,为SCI恢复创造持续的抗炎微环境。我们的体内实验表明,PBH@AGN 减少了疤痕组织的发生,促进髓鞘再生,并加速轴突再生。这种多药物递送系统结合了氨基胍的急性释放和 EV 的持续释放,对于协同管理 SCI 后具有挑战性的病理过程非常有效。















































 京公网安备 11010802027423号
京公网安备 11010802027423号