当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Variations on the Bergman Cyclization Theme: Electrocyclizations of Ionic Penta-, Hepta-, and Octadiynes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-09-25 , DOI: 10.1021/jacs.3c06691 Dominic A Sirianni 1, 2 , Xinli Song 2 , Salmika Wairegi 2 , Evan B Wang 2 , Sebastian A Mendoza-Gomez 2 , Adam Luxon 2 , Maxwell Zimmerley 2 , Ariana Nussdorf 2 , Michael Filatov 3 , Roald Hoffmann 4 , Carol A Parish 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-09-25 , DOI: 10.1021/jacs.3c06691 Dominic A Sirianni 1, 2 , Xinli Song 2 , Salmika Wairegi 2 , Evan B Wang 2 , Sebastian A Mendoza-Gomez 2 , Adam Luxon 2 , Maxwell Zimmerley 2 , Ariana Nussdorf 2 , Michael Filatov 3 , Roald Hoffmann 4 , Carol A Parish 2
Affiliation
|
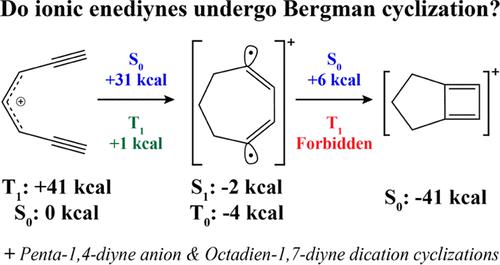
The Bergman cyclization of (Z)-hexa-3-ene-1,5-diyne to form the aromatic diradical p-benzyne has garnered attention as a potential antitumor agent due to its relatively low cyclization barrier and the stability of the resulting diradical. Here, we present a theoretical investigation of several ionic extensions of the fundamental Bergman cyclization: electrocyclizations of the penta-1,4-diyne anion, hepta-1,6-diyne cation, and octa-1,7-diyne dication, leveraging the spin-flip formulation of the equation-of-motion coupled cluster theory with single and double substitutions (EOM-SF-CCSD). Though the penta-1,4-diyne anion exhibits a large cyclization barrier of +66 kcal mol–1, cyclization of both the hepta-1,6-diyne cation and octa-1,7-diyne dication along a previously unreported triplet pathway requires relatively low energy. We also identified the presence of significant aromaticity in the triplet diradical products of these two cationic cyclizations.
中文翻译:

伯格曼环化主题的变体:离子五、七和辛二炔的电环化
( Z )-六-3-烯-1,5-二炔的伯格曼环化形成芳香族双自由基对苯炔,由于其相对较低的环化势垒和所得双自由基的稳定性而作为潜在的抗肿瘤剂而受到关注。在这里,我们对基本伯格曼环化的几种离子扩展进行了理论研究:五-1,4-二炔阴离子、七-1,6-二炔阳离子和八-1,7-二炔二价阳离子的电环化,利用具有单替换和双替换的运动方程耦合簇理论的自旋翻转公式(EOM-SF-CCSD)。尽管五-1,4-二炔阴离子表现出 +66 kcal mol –1的大环化势垒,七-1,6-二炔阳离子和八-1,7-二炔二价阳离子沿着先前未报道的三重态途径环化需要相对较低的能量。我们还发现这两种阳离子环化的三重态双自由基产物中存在显着的芳香性。
更新日期:2023-09-25
中文翻译:

伯格曼环化主题的变体:离子五、七和辛二炔的电环化
( Z )-六-3-烯-1,5-二炔的伯格曼环化形成芳香族双自由基对苯炔,由于其相对较低的环化势垒和所得双自由基的稳定性而作为潜在的抗肿瘤剂而受到关注。在这里,我们对基本伯格曼环化的几种离子扩展进行了理论研究:五-1,4-二炔阴离子、七-1,6-二炔阳离子和八-1,7-二炔二价阳离子的电环化,利用具有单替换和双替换的运动方程耦合簇理论的自旋翻转公式(EOM-SF-CCSD)。尽管五-1,4-二炔阴离子表现出 +66 kcal mol –1的大环化势垒,七-1,6-二炔阳离子和八-1,7-二炔二价阳离子沿着先前未报道的三重态途径环化需要相对较低的能量。我们还发现这两种阳离子环化的三重态双自由基产物中存在显着的芳香性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号