Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of Thiadiazole-Linked Covalent Organic Frameworks via Facile Linkage Conversion with Superior Photocatalytic Properties
Advanced Science ( IF 14.3 ) Pub Date : 2023-09-20 , DOI: 10.1002/advs.202304697 Shuailong Yang 1, 2 , Ziao Chen 1 , Lei Zou 1, 2 , Rong Cao 1, 2
Advanced Science ( IF 14.3 ) Pub Date : 2023-09-20 , DOI: 10.1002/advs.202304697 Shuailong Yang 1, 2 , Ziao Chen 1 , Lei Zou 1, 2 , Rong Cao 1, 2
Affiliation
|
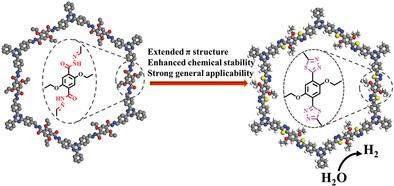
The establishment of facile synthetic routes to engineer covalent organic frameworks (COFs) with fully conjugated structure and excellent stability is highly desired for practical applications in optoelectronics and photocatalysis. Herein, a novel linkage conversion strategy is reported to prepare crystalline thiadiazole-linked COFs via thionation, cyclization, and oxidation of N-acylhydrazole bonds with Lawesson's reagent (LR). The as-prepared thiadiazole-linked COFs not only remain porosity and crystallinity, but enhance its chemical stability. Furthermore, thiadiazole-linked COFs are more favorable to lower exciton binding energy and promote π-electron delocalization over the whole reticular framework than N-acylhydrazone-linked COFs. Notably, the extended π-conjugation structure and decent crystallinity of the resulting TDA-COF are reflected by its higher photocatalytic H2 evolution rate (61.3 mmol g−1 in 5 h) in comparison with that (7.5 mmol g−1) of NAH-COF.
中文翻译:

通过简单的键合转化构建具有优异光催化性能的噻二唑连接的共价有机框架
建立简单的合成路线来设计具有完全共轭结构和优异稳定性的共价有机框架(COF)对于光电子和光催化的实际应用来说是非常需要的。在此,报道了一种新的键转换策略,通过劳森试剂(LR)对N-酰基肼键进行硫化、环化和氧化来制备结晶噻二唑连接的COF。所制备的噻二唑连接的COF不仅保持了孔隙率和结晶度,而且增强了其化学稳定性。此外,与N-酰腙连接的COF相比,噻二唑连接的COF更有利于降低激子结合能并促进整个网状框架上的π电子离域。值得注意的是,与NAH (7.5 mmol g -1)相比,所得TDA-COF具有更高的光催化H 2析出速率(5小时内61.3 mmol g -1 ),反映了所得到的TDA-COF的扩展π共轭结构和良好的结晶度。 -COF。
更新日期:2023-09-20
中文翻译:

通过简单的键合转化构建具有优异光催化性能的噻二唑连接的共价有机框架
建立简单的合成路线来设计具有完全共轭结构和优异稳定性的共价有机框架(COF)对于光电子和光催化的实际应用来说是非常需要的。在此,报道了一种新的键转换策略,通过劳森试剂(LR)对N-酰基肼键进行硫化、环化和氧化来制备结晶噻二唑连接的COF。所制备的噻二唑连接的COF不仅保持了孔隙率和结晶度,而且增强了其化学稳定性。此外,与N-酰腙连接的COF相比,噻二唑连接的COF更有利于降低激子结合能并促进整个网状框架上的π电子离域。值得注意的是,与NAH (7.5 mmol g -1)相比,所得TDA-COF具有更高的光催化H 2析出速率(5小时内61.3 mmol g -1 ),反映了所得到的TDA-COF的扩展π共轭结构和良好的结晶度。 -COF。































 京公网安备 11010802027423号
京公网安备 11010802027423号