Tetrahedron ( IF 2.1 ) Pub Date : 2023-09-21 , DOI: 10.1016/j.tet.2023.133660 Ho Suk Shin , Bong Ser Park
|
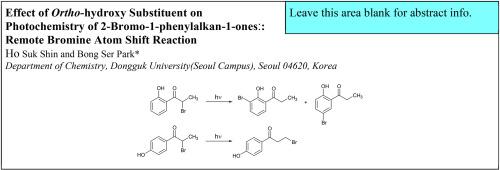
Irradiation of 2-bromo-1-(2-hydroxy)phenylalkan-1-ones forms ring-brominated phenyl ketones, where bromine moves from the α-position of the carbonyl to the phenyl ring. The unprecedented remote Br shift reaction occurs with regioselectivity favoring the ortho isomer, which originates from a hypobromite intermediate. The reaction has several advantages over other known methods for the preparation of the ring-brominated 1-(2-hydroxy)phenylalkan-1-ones: no poly-bromination, ortho-selectivity, no additives, use of eco-friendly light energy only, and no bromination at other active sites such as the benzylic position.
中文翻译:

邻羟基取代基对2-溴-1-苯基链烷-1-酮光化学的影响:远程溴原子转移反应
2-溴-1-(2-羟基)苯基烷-1-酮的辐照形成环溴化苯基酮,其中溴从羰基的α位移动到苯环。前所未有的远程 Br 变换反应发生,区域选择性有利于邻位异构体,该异构体源自次溴酸盐中间体。与其他已知的环溴化1-(2-羟基)苯基烷-1-酮的制备方法相比,该反应具有以下几个优点:无多溴化、邻位选择性、无添加剂、仅使用环保光能,并且在其他活性位点(例如苄基位置)没有溴化。








































 京公网安备 11010802027423号
京公网安备 11010802027423号